Biofuels and Bio Lubricants Production for Industrial Application; The Sudanese Experience
Babiker K. Abdalla1*
1 Professor of Chemical Engineering, Karary University, Sudan.
*Corresponding Author: Babiker K. Abdalla, Professor of Chemical Engineering, Karary University, Sudan, TEL: +249912389074 ; FAX: +249912389074;E-mail:babiker.k.abdalla@gmail.com
Citation: Babiker K. Abdalla (2018) Biofuels and Bio Lubricants Production for Industrial Application; The Sudanese Experience. SciEnvironm 1:110.
Copyright:© 2018 Babiker K. Abdalla, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received date: June 10, 2018; Accepted date: June 22, 2018; Published date: June 27, 2018.
Abstract
There has been a steady increase in the demand for environmentally friendly fuel; nearly 90% of the world's energy demand is met by the consumption of non-renewable fossil fuels. Due to the increasing energy demand throughout the world, the oil reserves are expected to last many years less than the projections made earlier. This coupled with the contribution of fossil fuels combustion to global warming and increasing mineral oil prices, has given momentum to the exploitation of renewal sources of energy, the production of biodiesel through transesterification of vegetable oils and animal fats being one of them. Also, the fermentation of biomass byproducts for the production of alcohols and the production of bio lubricants from non-edible oil and fats got more attention by number investigators. Sudan as an agricultural country with high population of animal livestock is an excellent candidate for the production of biofuels and bio lubricants. Research work; in Sudan, on converting the huge biomass by products into useful biofuels and bio lubricants was started by the end of the last century. It was started as small academic research and then immerged into a national campaign.
Keywords
Biofuels, Biodiesel, Jatropha Curcas Oil, Roselle Seeds Oil, Transesterification
Introduction
Biodiesel is defined as the mono-alkyl esters of fatty acids derived from vegetable oils or animal fats, in simple terms, biodiesel is the product you get when a vegetable oil or animal fat is chemically reacted with an alcohol to produce a fatty acid alkyl ester. Number of biomass byproducts types and non-edible oil seeds were used for the production of biofuels; i.e. Jatropha Curcas Oil [1], Roselle Seeds Oil [2], beside different types of spent frying Oils [3]. These different raw materials been treated and excellent results were obtained. High biodiesel yield (92.7 w/w %) from J. curcas oil (FFA<2%) [1]; was produced using one-step alkali based catalyzed transesterification. Beside was the production of the biolubricants using Sudanese Castor Seed Oil [4,5].
Biofuels Production
Ethanol is a relatively low-cost alternative fuel. It is considered to be better for the environment than gasoline. Ethanol-fueled vehicles produce lower carbon monoxide and carbon dioxide emissions, and the same or lower levels of hydrocarbon and oxides of nitrogen emissions. It burns with a smokeless blue flame that is not always visible in normal light. As the raw material of ethanol is farm based its production supports farmers and creates domestic jobs. And because ethanol is produced domestically, from domestically grown crops, it reduces dependence on oil and increases the nation’s energy independence. Worldwide fuel ethanol production is increasing day by day as per demand. For all these reasons; it is a great challenge for chemical engineers to produce ethanol in low cost.
Biodiesel can be produced from straight vegetable oil, animal oil/fats and tallow and waste oils. There are three basic routes to biodiesel production from oils and fats:
•Base catalyzed transesterification of the oil.
•Direct acid catalyzed transesterification of the oil.
•Conversion of the oil to its fatty acids and then to biodiesel.
Almost all biodiesel is produced using base catalyzed transesterification as it is the most economical process requiring only low temperatures and pressures and producing a 98% conversion yield. For this reason, only, this process will be described in this report. The Transesterification process is the reaction of a triglyceride (fat/oil) with an alcohol to form esters and glycerol. A triglyceride has a glycerin molecule as its base with three long chain fatty acids attached. The characteristics of the fat are determined by the nature of the fatty acids attached to the glycerin. The nature of the fatty acids can in turn affect the characteristics of the biodiesel. During the esterification process, the triglyceride is reacted with alcohol in the presence of a catalyst, usually a strong alkaline like sodium hydroxide. The alcohol reacts with the fatty acids to form the mono-alkyl ester, or biodiesel and crude glycerol. In most production methanol or ethanol is the alcohol used (methanol produces methyl esters, ethanol produces ethyl esters) and is base catalyzed by either potassium or sodium hydroxide. Potassium hydroxide has been found to be more suitable for the ethyl ester biodiesel production, either base can be used for the methyl ester. A common product of the transesterification process is Rape Methyl Ester (RME) produced from raw rapeseed oil reacted with methanol. The figure below shows the chemical process for methyl ester biodiesel. The reaction between the fat or oil and the alcohol is a reversible reaction and so the alcohol must be added in excess to drive the reaction towards the right and ensure complete conversion. Figure 1.
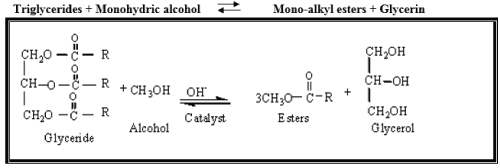
The products of the reaction are the biodiesel itself and glycerol. A successful transesterification reaction is signified by the separation of the ester and glycerol layers after the reaction time. The heavier, co-product, glycerol settles out and may be sold as it is or it may be purified for use in other industries, e.g. the pharmaceutical, cosmetics etc.
a. Jatropha Curcas Oil [JCO] [1]:
Jatropha curcas (J. curcas) seed oil is an attractive feedstock for biodiesel production, the seed has high oil content (36-40%), moreover, the properties of biodiesel produced agree with the American Society for Testing and Materials (ASTM) standard D6751-09, and the Committee of Standardization (CEN) standard EN 14214. High biodiesel yield (92.7 w/w %) from J. curcas oil (FFA<2%) was produced using one- step alkali based catalyzed transesterification. A two step Acid –base catalyzed transesterification is an effective and economical method for a high FFA (>2) J. curcas oil. In the first step (acid catalyzed esterification) FFA is converted to FAME which decreases the FFA to less than 2%, thus, acid pretreatment process is not affected by initial FFA. In the Second step (base catalyzed transesterification) high biodiesel yield (92.9 w/w %) is obtained.
b.Roselle Seeds Oil [ROS] [2]:
The outcome of this study has shown that biodiesel can successfully be produced from crude Roselle oil by acid or alkali-catalyzed transesterification reaction with alcohol methanol, which has been in this process due to its low cost in the presence of catalyst such as sodium and potassium hydroxide or sodium methylate. Roselle oil, which was extracted in the laboratory scale and analyzed. The result of the analysis has shown that the oil was clear, viscous, yellow in color, and has a density and oil percentage of 0.907 g/mL and 19.11 % respectively. The fatty acid composition analyzed by gas chromatography, has shown close agreement with the results obtained using the red clays. The produced biodiesel has been analyzed and compared with the standard biodiesel ASTM and found very promising from the obtained results. To determine the optimum transesterification reaction parameters (reaction temperature, reaction time, catalyst concentration and methanol to oil molar ratio), some reactions by differentiate one parameter while other parameters kept constant are made.
c. Spent Frying Oils [SFO] [3]:
The methyl ester was prepared from waste cooking oils with methanol to produce biodiesel was successfully performed with a maximum biodiesel yield of 92 wt% and methyl ester purity of 100 % for process, a maximum biodiesel yield of 87 wt% and methyl ester purity of 99,9 % for process. The optimal conditions and biodiesel yield may vary in terms of the quality of raw oils. The fuel properties of biodiesel derived from recycled oils, all met the ASTM standard and German Biodiesel Standard. The fatty acid composition of final biodiesel esters was determined by gas chromatography. Production of biodiesel from waste cooking oils for diesel substitute is particularly important because of the decreasing trend of economical extracted oil reserves and the environmental problems caused due to the use of fossil fuel. Waste cooking oil can be an important source for biodiesel production in Sudan as there is large quantity of waste cooking oil available. Use of waste cooking oil helps improve the biodiesel economics. The overall energy balance of bio-diesel is highly depending on the feedstock used and the utilization of by-products. However, in all cases the overall energy balance of biodiesel is positive.
Bioethanol from agricultural biomass [4-6]:
Ethanol (C2H5OH) is a clear, colorless, flammable chemical. It has been produced and used as an alcoholic beverage for several thousand years. Ethanol also has several industrial applications (e.g., in detergents, toiletries, coatings, and pharmaceuticals) and has been used as transportation fuel for more than a century. The production of ethanol by yeast is well-established technology. In order to produce a fuel Gasoline – ethanol blend is a fuel consisting primarily of gasoline along with a substantial amount of fuel ethanol. Massive Production of Molasses (by Product of Sugar Industry) in Sudan, (Sudan entering the age of green fuel production by inauguration of Kenana Ethanol Factory. It is the first of its kind in Africa, thus putting the country at an advanced level in this strategic industry). And one of problem statement several compelling issues drive a national effort to develop and improve technology to make biofuels. Our dependence on petroleum for fueling the transportation sector threatens our energy security, affects our environment, and weakens our economic. Developing the technology to produce and use biofuels will create transportation fuel options that can positively impact these issues and establish safe, clean, sustainable alternatives petroleum. The world is heading towards alternative sources of energy that are environmentally friendly and which reduce greenhouse gas emission hazards. Table 1.
Table 1: Kenana Ethanol production.
Million Liters
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
| 9,215,220 | 41,392,036 | 36,178,333 | 32,755,171 | 47,079,280 | 38,584,376 | 25,198,840 |
Data source: Kenana Ethanol Factories
Biolubricants Production [7-9]:
Sudanese Castor Seed Oil
Castor oil is a vegetable oil obtained from the castor bean (technically castor seed) that has an unusual structure and many uses. It is obtained by pressing and extraction the seeds of the castor plant, Ricinus communis. Sometimes called castor bean oil, this plant is not a member of the bean family. The castor-oil plant is easy to grow and is resistant to drought, which makes it an ideal crop for the extensive semi-arid regions globally. That area globally holds some twenty million hectares of appropriate land that could yield up to 1.5 tons of castor beans per hectare, compared to the global average of 750 kilos per hectare. Castor beans could become a farming alternative, providing income for over one hundred million people who suffer hunger in the poorest regions globally. Castor oil is a colorless to very pale-yellow liquid with mild or no odor or taste. Its boiling point is 313°C and its density is 961 kg/m3. It is a triglyceride in which approximately 90 percent of fatty acid chains are ricinoleate. Oleate and linoleates are the other significant components. The castor seed contains ricin, a toxic protein. Heating during the oil extraction process denatures and inactivates the protein. However, harvesting castor beans may not be without risk. Allergenic compounds found on the plant surface can cause permanent nerve damage, making the harvest of castor beans a human health risk. India, Brazil, and China are the major crop producers, and the workers suffer harmful side effects from working with these plants. These health issues, in addition to concerns about the toxic byproduct (ricin) from castor oil production, have encouraged the quest for alternative sources for hydroxyl fatty acids. Alternatively, some researchers are trying to genetically modify the castor plant to prevent the synthesis of ricin. With the world becoming more environment conscious and with increasing replacement of synthetic products with naturally derived products, castor oil-based derivatives could find increasingly attractive markets worldwide. So, because of this it is need of clean environment friendly [7]. The percentage oil content of castor seed was found to be 31.98% of the total weight of 910g. As such a satisfactory result cannot be achieved by solvent extraction process using laboratory Soxhlet apparatus. The castor oil produced in this research work was analyzed for specific gravity, Viscosity at 40 and 100°C, acid value, saponification value and iodine value. Most of the values comply with the standard specified by ASTM (1952). The oil is of good quality and could be recommended suitable for industrial usage. Vegetable oil is much desired for its application as a lubricant in metal forming process and internal combustion engines, because it is a renewable resource and has high biodegradability compared to mineral oil.
Conclusions
These studies demonstrated that the Sudanese experience in the production of biofuels can successfully be produced from crude vegetable oils by alkali-catalyzed transesterification reaction with methanol in the presence of a catalyst potassium hydroxide (KOH). These processes can be considered alternative fuels, which will reduce pollution and protect the environment. Transesterification reaction for the production of biodiesel was found to be very promising from the results obtained. Beside the huge biomass produced from the sugar industries and other agricultural project; which will supply large volume of bioethanol (Gasohol) and biolubricants.
References
- Maha AA Abdelrahman, Atif AA Yassin, Esmail H Hussein, Babiker K Abdalla (2014) “Stability of Jatropha Curcas Biodiesel [JCB]”. Pinnacle Engineering & Technology V2014: 6.
- Nadir B Ahmed, Babiker K Abdalla, Ibrahim HM Elamin, Yasir Ibrahim Elawad (2016) “Biodiesel Production from Roselle Oil Seeds and Determination the Optimum Reaction Conditions for the Transesterification Process”. Int. J. of Eng. Trends & Tech. 39: 105-111.
- BK Abdalla, FOA Oshaik (2013) “Base-transesterification process for biodiesel fuel production from spent frying oils”. J. of Agri. Sci. 4: 85-88.
- Gibreel M I (2007) “Utilization of Gasoline–Ethanol Blends (Gasohol) as an Alternative Fuel”, M. Sc., Karary University, Sudan.
- Nadir B Ahmed (2011) “Natural Gas Synthesis For Production Of High Octane Number Gasoline”, M. Sc. Thesis, University of Gezira, Sudan.
- Elgaily M. I. Adam (2011) “Simulation and Optimization of Biochemical Process to Produce Bioethanol”, M. Sc., Karary University, Sudan.
- Abdelaziz AIM, Elamin IHM, Gasmelseed GA, Abdalla BK (2016) “Conditioning of Castor Oil to be Use as Lubricant”. Inter. J. of Chem. and Nat. Sci. 4: 409-411.
- Abdelaziz AIM, Elamin IHM, Gasmelseed GA, Abdalla BK (2014) “Extraction, Refining and Characterization of Sudanese Castor Seed Oil”. J. of Chem. Eng. 2: 1-4.
- Khalil AS, Rahim AA, Taha KK, Abdalla KB (2013) “Characterization of Methanolic Extracts of Agarwood Leaves”. J. of App. and Ind. Sci. 1: 78-88.
