Can We Personalize The Management of Obesity and Morbid Obesity Implementing Genetic, Metabolic and Hormonal Markers?
Giath Alshkaki Osman1*
1 Infinity Surgical Associates, Alexandria, Virginia, USA.
*Corresponding Author: Giath Alshkaki Osman, Infinity Surgical Associates, Alexandria, Virginia, USA, TEL:(703) 888-0731 ; FAX:(703) 888-0731;E-mail:alshkaki@infinitysurgery.com
Citation: Giath Alshkaki Osman (2018) Can We Personalize The Management of Obesity and Morbid Obesity Implementing Genetic, Metabolic and Hormonal Markers? Arch Mol Med & Gen 1:105.
Copyright: : © 2018 Giath Alshkaki Osman, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received date: October 24, 2018; Accepted date: November 05, 2018; Published date: November 08, 2018.
Morbid Obesity
National & International crisis in the form of an epidemic
Over the passed decade the number of over weight individuals increased to more than 60%
Morbid Obesity BMI 40 Or 35 (BMI kg/m2)
About 500,000 procedures for MO in USA. Figure
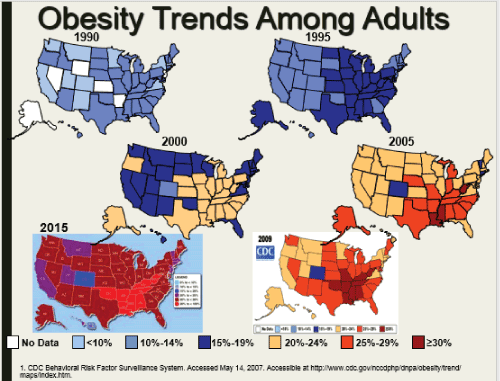
Figure 1
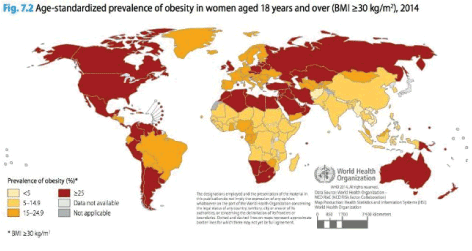
http://www.takepart.com/article/2015/01/24/most-obese-countries-maps/
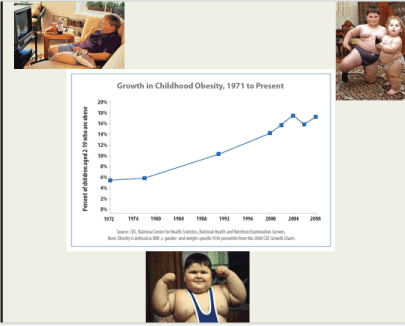
Figure 3
Summary of an Epidemic
68% of U.S. adults are overweight or obese [1]
Total medical cost for obesity in 2008 was $147 billion [2]
9.1% of increased annual medical spending was associated with obesity [2]
Obese patients had 42% higher per capita medical spending than normal weight individuals [2]
Obese patients had 80% higher per capita spending for prescription drugs [2]
What is Morbid Obesity?
•False: Over eating
•True: Lifelong and progressive
•Questionable:
–Multifactorial disease of excess fat storage with a genetic basis?
–Environmental influence?
Metabolic Syndrome
Figure 4. Metabolic Syndrome is a multi-factorial disease created by a complex interaction betweeen genetic, metabolic, and environmental factors.
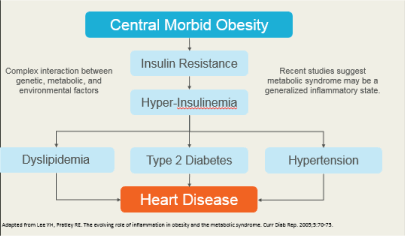
Figure 4
Thrifty Genes Contribute to Morbid Obesity
•Genetic factors can account for 20 to 80 percent of a person’s tendency to develop obesity [3].
•These “thrifty genes” are designed to protect us from starvation by allowing us to store large amounts of energy in the form of fat when food is abundant [4].
•This is the first time in human history that food has been so abundant [4].
Thrifty genes, defined in a group of native americans, conferred a survival advantage to humans by allowing humans to gorge on food during abdundant times and store this energy as fat. Humans could then survive long winters or trips by relying on this stored food. The modern day abundance of food, especially high fat and high sugar foods, creates a situation where these genes become a source of disease instead of a survival advantage. Figure 5,6.
Figure 5
Figure 6
Complex Hormonal Control of Weight Homeostasis
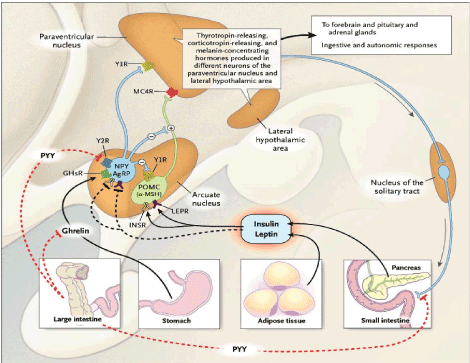
Figure 7
Ghrelin
•Hormone secreted predominantly by gastric cells; recognized in 1999 as a mediator of growth-hormone release [5]
•Increases an hour or two before a meal and goes into a “trough-like” level after eating [6]
•Weight loss of 17% of body weight from dieting is associated with a 24 percent increase in the 24-hour ghrelin profile [6]
•Activated by the presence of dietary lipids [7].
Ghrelin, recently discovered in 1999 by Dr. Cummings, is a hormone created in the cells of the stomach. Ghrelin acts on the midbrain, driving the feeling of hunger. As ghrelin levels increase, the level of hunger experienced increases. Weight loss from dieting is associated with increases in ghrelin levels and the ghelin levels remain elevated until they regain any lost weight. The hormonal drive to eat becomes increasing strong, leading to diet failure. Weight loss from gastric bypass surgery is associated with a decrease in ghrelin levels. The impact of Ghrelin in weight loss is still being studied. Figure 8.
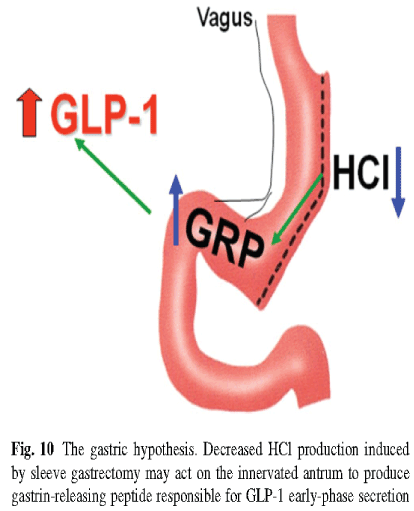
•Reduced Serum Ghrelin (70-90%)
•Vagal stimulation (increased gastric pressure)
•Increased gastric emptying
•rapid transport of food bolus to distal gastrointestinal tract (“ileal brake”)
•Increased GLP-1 and PYY 3-36 affecting neuropeptide Y (inhibits release of neuropeptide Y from the arcuate nucleus)
•PYY induces satiety and decreases food intake
Research project budget for obesity
$15 billion Prevention and Public Health Fund. Designed to support a range of programs for prevention, wellness and health activities including research and health screenings.
http://www.depts.ttu.edu/hs/obesityresearch/funding.php
Research project topics for obesity
•Liver changes with Obesity.
•Genetic studies on subcutaneous and visceral fat.
•Hormonal changes with Obesity.
•Intestinal Bacteria association with Obesity.
•Testing Neural Mechanisms for feeding inhibition.
•(Obesity Driven Primarily by Hyperphagia.
•Intake Inhibition mechanisms.
•Leptin, Ghlein, GLP1, Oxytosin Receptor Signaling
•Central Neurotransmitter System, Energy balance.
delete or combine with the slide before Figure 10.
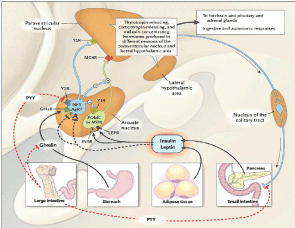
Figure 9

PRE-study Data Analysis
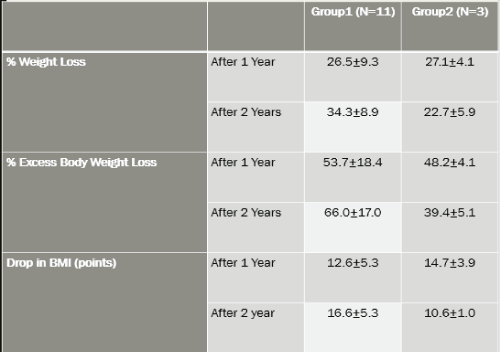
•A total of fourteen obese subjects who underwent the sleeve gastrectomy participated in this study.
•Group 1 consists of eleven subjects (2 males and 9 females) aged between 28 and 68 years, lost weight and maintained it 2 years out.
•Group 2 consists of three subjects (3 females) aged between 39 and 43 years lost weight after surgery then gained weight with in the 2 years follow up.
The data collected previous to the main experiment Figure 11.
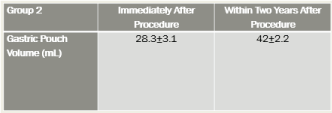
Post-Procedure Results Figure 12.
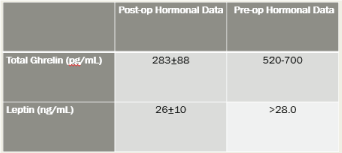
Group 2 Post-Procedure Hormonal Results Figure 13.
Current Bariatric Surgical Procedures
•Laparoscopic Adjustable Gastric Banding
•Sleeve Gastrectomy
•Banded Sleeve Gastrectomy
•Roux-en-Y Gastric Bypass
•Duodenal Switch / Biliopancreatic Diversion
•Intra Gastric Balloons
•Vagal Nerve Stimulators
•Endoscopic Procedures
Figure 14. Group 1-lost and maintained weight loss. Group 2-lost weight for one year then slowly gained the weight back.
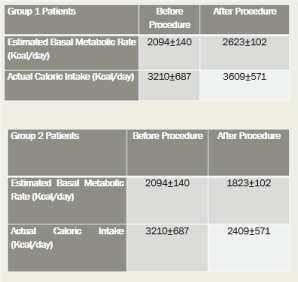
Figure 14
Statments
• Two key foundations for management: surgical and medical management
• Still a recurrence of failed treatment and of diabetes in patients
• We have all noticed a friend or family member who is the ‘lucky one’ because he or she eats what ever they like without weight gain
What is their secret? What is this unique ability to keep their weight in a healthy range? Can we discover their secret?
Research Strategy Aims
Specific Aim 1: Measure levels of ghrelin, leptin, obestatin, insulin, glucagon, T3, T4, TSH, parathyroid hormone (PTH), basal metabolic rate, and body temperature in four groups of subjects in response to four different meal types and identify the mechanisms that cause the changes between the study groups.
Specific Aim 2: Investigate DNA methylation, microRNA, mRNA, S-Adenosyl methionine (SAMe), methionine, vitamin B12, vitamin B6, folate, homocysteine, and methylmalonic acid plasma levels, and inflammation processes caused by metabolic dysregulation and identify the cellular mechanisms that control the rate and expression of ghrelin, leptin, and obestatin.
Specific Aim 3: Develop a computer model that can be used to predict response to prevention and treatment of obesity and associated health consequences.
Aim 3: Computer Module
• Purpose 1: To design modeling and simulation techniques to investigate the interactions of ghrelin with endocrine and metabolic hormones in obesity progression.
• Recently: Attention was focused on ghrelin’s role in regulating body weight. However, the precise role of ghrelin remains elusive.
• Purpose 2: To investigate the associations of genetics and hormonal mechanisms in patients who gain weight after substantial weight loss following sleeve gastric surgery.
• The computer model will attempt to correlate the genetic expression and regulation of ghrelin and its receptors to the other outcomes of the study.
Hypothesis
Hypothesis 1: Ghrelin and leptin plays a role in toning down the surge of insulin and glucagon, and obestatin regulates the excess rate of their activities
Hypothesis 2: There may be another hormonal factor playing a role in this regulatory mechanism
Hypothesis 3: These mechanisms allow human subjects with steady weight and normal activity levels to increase their basal metabolic rate by burning carbohydrates while sleeping. Discovering such regulatory mechanism will be the key factor for the treatment of obesity and related comorbidities.
We hypothesize that ghrelin, leptin, obestatin, thyroid hormones and their genetic regulations interact through unknown and untested regulatory mechanisms that affect the overall basal metabolic rate during sleep.
SELECTION OF FOUR GROUPS
Group 1: 10 healthy subjects with BMI 19-25, no previous weight loss treatments and regular domestic activities, no calorie restrictions, and have maintained normal weight for at least 5 years.
Group 2: 10 subjects with a BMI 30-50, no previous bariatric surgery, and have not been on any weight reduction medication or dietary regimen in the last one year prior to the study participation.
Group 3: 10 subjects who had pre-LSG (laparoscopic sleeve gastrectomy) BMI 30-50 and lost at least 45%-50% of their excess body weight, have been at least 16 months post-surgery, and have maintained their weight loss within 5%.
Group 4: 10 subjects with a pre-LSG BMI 30-50 and who experienced initial weight loss of at least 45%-50% of extra body weight following LSG but subsequently gained back at least 15% of the weight.
and analyze data from four groups each of 10 adult human subjects, 25-60 years of age.
Figure 15. This is the standard procedure for each patient. It was very important to retain a consistency in this protocol. Figure 16.
Figure 15
Figure 16
- Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999-2008. JAMA 303: 235-241. [crossref]
- Finkelstein EA, Trogdon JG, Cohen JW, et al. (2009) Annual medical spending attributable to obesity: Payer- and service-specific estimates. Health Affairs 28: w822-w831.
- Maes HHM, Neale MC, Eaves LJ (1997) Genetic and Environmental Factors In Relative Body Weight And Human Adiposity. Behavior Genetics 27: 325-351.
- Cummings DE, Schwartz MW (2003) Genetics and pathophysiology of human obesity. Annu Rev Med 54: 453-471. [crossref]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, et al. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656-660. [crossref]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, et al. (2002) Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623-1630. [crossref]
- Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, et al. (2009) GOAT links dietary lipids with the endocrine control of energy balance. Nat Med 15: 741-745. [crossref]
