Animal Dietary Manipulation and Their Manure Management Systems to Mitigate Green House Gas Emissions: A review article
Negasu Gamachu Dinsa1*, Reta Adisu Abeba2
1Oromia Agricultural Research Institute (IQQO), Haro Sabu Agricultural Research Centre, Haro Sabu, Oromia, Ethiopia.
2Dale sadi Woreda livestok and fisher development office, animal health officer.
*Correspondence Author: Negasu Gamachu Dinsa, Oromia Agricultural Research Institute (IQQO), Haro Sabu Agricultural Research Centre, Haro Sabu, Oromia, Ethiopia, Tel: +251-912-973613, +251-575-560611; Fax: +251-912-973613; E-mail: negasugamachu@gmail.com
Citation: Negasu Gamachu Dinsa, Reta Adisu Abeba (2020) Animal Dietary Manipulation and Their Manure Management Systems to Mitigate Green House Gas Emissions: A review article. Allergy drugs clin immunol 4: 120.
Copyright: © 2022 Negasu Gamachu Dinsa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received date: April 07, 2022; Accepted date: April 20, 2022; Published date: April 22, 2022.
Abstract
Animal production is a significant source of greenhouse gas emissions worldwide. This t paper was reviewed on the method of animal dietary manipulation and their manure management practices for mitigating methane and nitrous oxide, i.e. Non-carbon dioxide greenhouse gas emissions from enteric fermentation and animals manures. The chemical composition of animal dietary is an important factor which affects rumen fermentation and greenhouse gas emission by the animals. Feed additives have been comprehensively studied in vitro and in vivo for their methane mitigating potential. The use offodder trees has been developed through the process of pelleting; Leucaena leucocephala leaf pellet, mulberry leaf pellets and mangosteen peel and/or garlic pellets, can be used as good sources of protein to supplement ruminant feeding. This approach could help to decrease rumen protozoa and methanogens and thus mitigate the production of methane gas. Greenhouse gas mitigation from manure should be targeted at farm specific management practices. Anaerobic bio-digesters, covered lagoons or manure storages with methane flaring systems or small electricity generators, land application at appropriate time are gaining popularity as viable technologies to abate greenhouse gas emissions from manure storage. Considerable additional research is still needed in order to use both conventional and non-conventional feed resourcestheir potential to affect greenhouse gas emission by the animals. Manure greenhouse gas emission mitigation practices should be evaluated for co-benefits & pollution swapping effects at a whole farm levels.
Keywords
Green House Gas, Animal Dietary, Manure Management Systems, Mitigation
Introduction
Livestock is one of the fastest growing sub-sectors of agriculture: a doubling of demand for animal source foods is expected for developing countries and a 70% increase for the world as a whole [1]. The livestock production sector is a key contributor to environmental challenges at local, regional and global scales [2,3]. As the name implies, the gases that assist in capturing heat in the atmosphere are termed as greenhouse gases (GHGs).This GHG emitted from the agricultural sector contribute to total global radiation is about 25.5% and over 60% of anthropogenic sources [4]. Livestock production operations contribute both directly and indirectly to climate change through the emissions of greenhouse gases such as carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O).TheCH4, CO2, and N2O are considered as direct greenhouse gases. The indirect GHGs include carbon monoxide (CO), oxides of nitrogen (NOx), and non-methane volatile organic compound (NMVOCs) [5].
Main sources of pollutant gases are broadly classified as natural (geogenic and biogenic) and anthropogenic. From those three classification natural Biogenic sources of GHGs, such as those contained in grass, hay, silage, and grains are a major part of bovine diets and are emitted from these biogenic sources during fermentation of starches, lipids, and proteins in the digestive system of cattle (enteric fermentation) and later in the feces and urine [5]. Livestock production accounts for 18% of GHG emissions that cause global warming [6]. The livestock sector is estimated to contribute 14.5% of all global anthropogenic greenhouse gas (GHG) emissions (Henderson, et al. 2017). The 3 main GHG are carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), and their emissions are usually expressed on a CO2-equivalent (CO2eq) basis to represent their global-warming potential in the atmosphere.
Feed production, enteric fermentation and manure management are major sources of GHG from the livestock sector [7]. As European commission science for environmental policy reported that, Globally, there is the potential to reduce GHG emissions from the livestock sector by as much as 2.4 metric gigatonnes of CO2 equivalent emissions every year (GtCO2-eq yr−1) (Henderson, et al. 2015).
Reducing the increase of GHGs emissions from agriculture, especially from livestock production should therefore be a top priority, because it could limit global warming substantially and faster [8]. The development of management strategies to mitigate CH4 emissions from ruminant livestock is possible and desirable. The largest carbon equivalent emissions were from CH4 (72.6%), N2O (24%) and CO2(3.4%) which indicated the need to improve livestock and manure management systems under smallholder agriculture. New dietary strategies are in place in some developed countries for the reduction of CH4emissions from ruminants by manipulating ruminal fermentation directly to inhibit methanogens and protozoa or to divert hydrogen ions away from methanogens. In developing countries, change in feeding systems, breed selection, good animal husbandry and improved take-off were identified as viable options for the reduction of greenhouse gas emissions. However, existing mitigation strategies for CH4emissions in dairy production, such as the use of high quality forages and increased use of grains were recommended management practices [9]. Therefore, the Objectives were to review and illustrate up to date information on manure management systems and animal dietary manipulation to mitigate greenhouse gas emission from live stocks.
Literature Review
Greenhouse gas emission in livestock sectors
Livestock production represents the largest anthropogenic source of methane (CH4) and nitrous oxide (N2O) [7] and contributes a range of critical environmental problems [10], including greenhouse gas (GHG) emissions [11], ammonia (NH3) emissions and alteration of nitrogen cycles [12], land and water use, [13] and miss use of antibiotics leading to anti-microbial resistance. Relative to ruminants, however, monogastric animals are minor emitters of GHG. The IPCC [14] assumes enteric CH4 emission factors for pigs at about 1.2 to 2.8 percent of the emission factors for cattle [1.5 vs 53 (beef or growing cattle) or 128 kg CH4/head per year (high-producing North American dairy cow)]. Recent estimates place GHG emissions from pigs at about 9.5 percent of the total emissions from livestock [7] and according to the same authors, the contribution of poultry to the global livestock GHG emissions is around 9.7 percent.
Figure 1: Total emissions from the global livestock sector, by main animal species and commodities ( Mton CO2-eq 2005) [7].
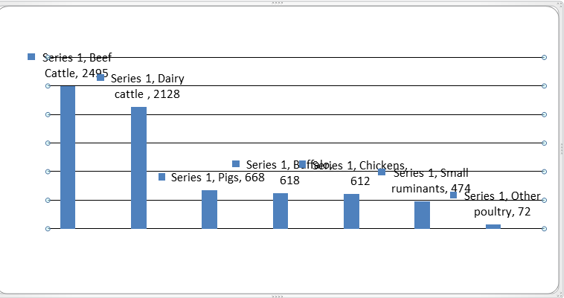
Domestic non-ruminant herbivore animals (horses, donkeys, mules, hinnies) produce enteric CH4 as a result of fermentation processes in their hindgut. However, hindgut fermenters do not produce as much CH4 per unit of fermented feed as ruminants, perhaps as a result of availability of hydrogen sinks other than CH4 (Jensen, 1996)The IPCC [14] assumes enteric CH4 emissions from horses at 18 kg/head per year (compared with 128 kg for a high-producing dairy cow of similar body weight). With the world horse population standing at around 58.8 million (FAOSTAT, 2010), global enteric CH4 emissions from horses can be estimated at about 1.1 Mt CH4/yr. Assuming a GWP of CH4 at 25, enteric CH4 emissions from horses represent 26.5 Mt CO2-eq/yr, which is around 0.6 percent of the global GHG emissions from cattle [7].
Livestock produce large quantities of manure rich in nitrogen and organic matter that contribute considerably to global emissions of NH3 and GHGs [15]. Approximately 40% of the global anthropogenic NH3 and N2O emissions are associated with livestock manures [16,17].
Manure production varies by animal type and is proportional to the animal’s weight and feed intake. Based on very recent report done by [9]Socioeconomic survey done on use and management of manure by smallholder farmers and their effect on the environment under farmers conditions at Adaa district, Ethiopian, the amount of CH4produced was estimated at 328.3 kg per year per household (Table 1).
Table 1: Average household methane and nitrogen emissions Adaa district, Ethiopia.
|
Species |
Average number per house hold |
Methane emission (kg/yr) |
Total |
N from manure(kg/yr) |
|
|
Fermentation |
Manure |
||||
|
Cattle |
8.3 | 265.6 | 8.3 | 273.9 | 318.72 |
|
Shoats |
4.3 | 21.5 | 0.73 | 22.2 | 46.44 |
|
Equines |
2.1 | 29.4 | 2.67 | 32.1 | 83.16 |
|
Poultry |
7.6 |
- |
0.14 | 0.14 | 3.76 |
|
Total |
22.3 | 316.5 | 11.84 | 328.3 | 452.1 |
Global warming potential (GWP) of green house gas emission:
The major global warming potential (GWP) of livestock production worldwide comes from the natural life processes of the animals. The main sources of GHGs during livestock production are CH4, N2O and CO2 (David W. Smith, 2014). CH4 emitted from enteric fermentation and manure management about 30% of total livestock CO2-eq emissions [18]. As [9] cited in ([14], the global atmospheric concentration of CH4has increased from a per-industrial value of about 715 ppb to 1 732 ppb in the early 1990s, and 1 774 ppb in 2005. According to [4] CH4was 25 times more powerful than CO2in global warming potential (GWP). Emissions of 1 million metric tons of CH4are equivalent to the emissions of 25 million metric tons of CO2called “equivalent CO2” (CO2e). This is the concentration of CO2that could cause the same level of radioactive force and concentration of greenhouse gases [19]. The second one is N2O from manure about 25% [4,11]and CO2 from deforestation and degradation of pasture (about 35%) [11] Table 1 describes the salient features of the three those major GHGs. The third one is CO2 it Sources are from the livestock farm include, animal respiration, and microbial respiration in the manure. Carbon dioxide can also be assimilated on the farm via carbon fixation [20]. Table 2.
Table 2: Global warming potential (GWP) of the GHGs.
|
GHG sources |
Chemical Formula |
Lifetime (years) |
Radioactive efficiency (W m-2ppb-1) |
Global Warming Potential |
References
|
|
Carbon dioxide |
CO2 |
100-120
|
|
1 |
IPCC, 2007). |
|
Methane |
CH4 |
10-12 |
|
23-25 |
Smithson, 2002; IPCC, 2007). |
|
Nitrous Oxide |
N2O |
114 |
|
298-310 |
IPCC, 2007). |
Feed associated option to reduce GHG emitted from ruminant animals
Animal dietary manipulation: The chemical composition of diet is an important factor which affects rumen fermentation and methane emission by the animals. Methane production was significantly lower in the sheep fed on green sorghum and wheat straw in the ratio of 90:10 as compared to where the ratio was 60:40 (31.5vs46.91/kg). Improvement in the digestibility of lignocelluloses feeds with different treatments also resulted in lower methanogenesis by the animals [21]. Wheat straw treated with urea (4kg urea par 100kg DM) or urea plus calcium hydroxide (3kg urea+3 kg calcium hydroxide per 100kg DM) and stored for 21 days before feeding, reduced methane emission from sheep. The treatment of straw with urea and urea molasses mineral block lick caused a reduction of 12-15% methane production and the molar proportion of acetate decreased accompanied with an increase in propionate production [21]. The absolute amount of CH4 formed per animal on different diets is related to characteristics of the feed in complex ways including the nature and amount of feed, the extent of its degradation, and the amount of H2 formed from it [22].
Feeding diets based on non-structural carbohydrates: It is well known that feeding diets with higher grain contents result in less methane per kg dry matter (DM) compared with forage-based diets [23]. The inclusion of starchy feeds can lower rumen pH and enhance the production of propionate resulting in a lower methane release [24]. The percentage of gross energy intake converted to methane of diets consisting primarily of grains is typically less than 4% compared with 6.5% or more for diets consisting mainly of forages [25]. Using high contents of concentrates in diets of dairy cattle is however limited, because rumen pH, milk quality and animal health are negatively affected by an excessive concentrate content in the diet [26]. FAO, [27] cited in the Noziere et al. (2010), , estimated that VFA molar proportions (acetate, propionate, butyrate) would average, respectively, 66, 17 and 14 mol/100 mol for NDF and 41, 44 and 12 mol/100 mol for starch. Thus, it is generally believed that higher inclusion of grain (or feeding forages with higher starch content, such as whole-crop cereal silages) in ruminant diets lowers enteric CH4 production. Beauchemin et al. [28] estimated that implementing extensive forage feeding for growing beef cattle would substantially increase GHG intensity (6.5 percent increase). Similarly, Pelletier et al. [10] reported 30 percent higher total GHG emissions for pasture-finished cattle compared with cattle in a grain-based feedlot system.
Ruminal bypass: The use of feed stuffs with nutrients that are known to be digested in the small intestine instead of being fermented in the rumen constitutes a further opportunity to reduce rumen methanogenesis. [29] Bypass substances such as starch in maize or sorghum are to a lesser degree rumen degradable compared to other grains [30], and deliver therefore less hydrogen as substrate for rumen methanogenesis. As pointed out by Leberl (2009) bypass protein seems less important compared to bypass starch, because it was supposed that the population of Achaea remains unaffected by bypass protein, as long as the rumen microbes are not undersupplied with nitrogen.
Feed additives: Feed additives have been comprehensively studied in vitroand in vivofor their methane mitigating potential. Due to their different origin and chemical structures, it is assumed that they have different modes of action [31,32]. However those different feed additives can be classified mainly to one of the following groups: lipids, ionophores, secondary plant compounds and organic acids. Lipids and secondary plant compounds can also be naturally feed ingredients, e.g. diets consisting of sun flower seeds or clover. Table 5 gives an overview of feed additives and their presumed mode of action to reduce rumen methanogenesis.
Secondary plant metabolites: The term plant secondary metabolite is used to describe a group of chemical compounds found in plants that are not involved in the primary biochemical processes of plant growth and reproduction [21]. More than 200,000 defined structures of plant secondary compounds have been identified [33]. Recently, Bodas et al. (2008) screened 450 plants for their possible anti-methanogenic effects. Thirty-five plants decreased methane production by more than 15%, and 6 of these plant additives i.e. Carduus pycnocephalus, Populus tremula, Prunus avium, Quercus robur, Rheum nobile and Salix caprea decreased methane production by more than 25%, with no adverse effects on digestibility, total gas and VFA production. Some of these metabolites, which have been shown to suppress methane production, are reviewed here. Some these PSM can generally be classified into four major groups: saponins, tannins, EO and others compounds are the discussed in this paper.
Tannins: Tannins occur in many plants suitable for feeding, especially in the tropics and subtropics. Many type of forages known to contain CT or tannin extracts have been shown to decrease methane production both in vivoand in vitroconditions. Through feeding of tanniferous browse plants, it has been found to decrease methane production, which is beneficial for sparing of energy loss as methane. The addition of tannins from Acacia mearnsiie.g. reduced enteric CH4 formation in sheep [34] and dairy cows [35]. Different types of tannin containing forages decreased CH4 emission in vitro[36,37]. [38], reported that Quebracho tannins inhibited the methane production linearly (13–45%) with increasing doses (5–25% of substrates). Quebracho tannin sample containing 7.62% HT and 3.67% CT inhibited methanogens at 50 g/kg of substrates and further inhibitory effect was noted at 250 g/k of substrates [38].
Tannin concentrations higher than 5% in diets might negatively influence feed intake [39], due to reduced palatability. Several studies reported a negative influence of tannins on feed digestibility [35,37,40]. Protein degradation in the rumen is affected by tannins due to formation of tannin-protein-complexes (Mueller-Harvey, 2006). Also Patra and Saxena, [33] cited in the [41] addition of Quebracho tannins in the diet of sheep at 0, 0.5, 1.5 and 3 g/kg BW (equal to 0, 28, 83 and 166 g/kg DM) did not affect feed intake up to 1.5 g/kg BW, but significantly decreased at the highest dosage. Similarly, Quebracho CT up to 2% of DM had no influence on feed intake in cattle [42]. Due to their antimicrobial action on rumen microbes, tannins may also decrease fiber degradation [33].
Saponins:[43] reported a decreased CH4 formation when feeding saponins to sheep (0.13g/kg diet). The CH4 mitigating effect of saponins results predominantly from decreased protozoa populations. Further, a decreased CH4 production rate by methanogens might be possible (reviewed by [33]. Chemically, saponins are a group of high molecular-weight glycosides in which saccharide chain units (1–8 residues) are linked to a triterpene (triterpene saponins) or steroidal (steroid saponins).A number of studies have reported reduce of methane through an inhibitory effect of saponins on methanogens in the rumen (Table 3).
Table 3: Effects of saponins or saponin-containing plants on methane production and fermentation in the rumen.
|
Saponins |
Test system (duration) |
Dosage |
Substrate/feed |
Methane inhibitiona |
References |
|
Acacia concinna pod extracts (ethanol and methanol) |
HGT (24 h |
Ethanol and methanol extracts of 0.5 ml/ 30 ml (0.2 g substrate) |
Wheat straw: concentrate (1:1) |
||
|
Knautia arvensis leaves extract (saponins 82.4%) |
HGT (24 h) |
Substrate |
Hay: concentrate (1:1) |
||
|
Serum bottle (24 h) |
Substrate |
Barley silage: concentrate (51:49) |
||
|
Serum bottle (24 h) |
Substrate |
Barley silage: concentrate (51:49) |
||
|
Quillajasaponaria extract(Mitsuba Trading, Japan; 5–7% saponins) |
Continuous culture fermentationvessels (24 h) |
medium or 92.0– |
Oat hay: concentrate (1:1) |
No effect |
Pen et al. (2006) |
|
Sapindus saponaria fruits (saponins, 120 g/kg) |
Rusitec (10 days) |
Diet |
Meadow grass: Arachis pintoi hay: barley straw (56:22:11) |
20% |
Hess et al. (2003a |
|
Sarsaponin (DK international,USA |
Sheep (15 days) |
Orchard grass silage: concentrate (70:30) |
|||
|
Sesbania sesban leaves |
HGT (24 h) |
Substrate |
Hay: concentrate (32:68) |
- |
|
|
HGT (24 h) |
Substrate |
Hay: concentrate (1:1) |
||
|
Tea saponins (60% saponins) |
HGT (24 h) |
Substrate |
Grass hay: corn (50:50) |
13% |
|
|
Trigonella foenum-graecum seeds (fenugreek) |
HGT (24 h) |
Substrate |
Hay: concentrate (32:68) |
||
|
HGT (24 h) |
Substrate |
Hay |
||
|
Yucca schidigera extract (Mitsuba Trading, Japan; 8–10% saponins) |
Continuous culture fermentation vessels (24 h) |
medium or 93.6– |
Oat hay: concentrate (1:1) |
Pen et al. (2006);2008 |
|
|
Serum bottle (24 h) |
Substrate |
Barley silage: concentrate (51:49) |
Source :reviewed by [33]. a=Inhibition of methane production compared with control (without phytochemicals) on volume basis. HGT = hohenheim gas test system. 24 h=twenty four hour.|
Methanogen populations were decreased in the presence of Sesbaniasesbansaponins by 78%, fenugreek saponins by 22% and Knautia saponins by 21% in the in vitro fermentation media with the rumen liquor collected from cattle [44]. Saponin-extracts from Yucca schidigera(sarsaponins; steroidalsaponins) and Quillaja saponaria (triterpenoid type saponins) or these plants as such have been examined in different laboratories, which have been demonstrated to reduce methanogenesis both in vitro (Takahashi et al., 2000; Pen et al., 2006, 2008; and in vivo studies [43,45,46] (?liwi?ski et al., 2002); Pen et al.,2007). [45] and Wang et al. [43] reported that feeding of sarsaponins for 25 days (35% saponins) to sheep reduced methane production by 7.1% (0.12 g/kg diet) and 15.5% (0.13 g/kg diet), respectively.
Supplementation of essential oils (EO):[33] cited in the [47], essential oils (EO) are obtained by steam distillation from different plants. Chemically, they are variable mixtures consisting principally of terpenoids [48]. Many of them appear to decrease methanogenesis in vitro and in vivo. The CH4 mitigating effect of essential oils might be due to suppression of methanogens and hydrogen producing microorganisms.
Figure 2: Essential Oils (ppm) inhibit Methane gas (ml) production.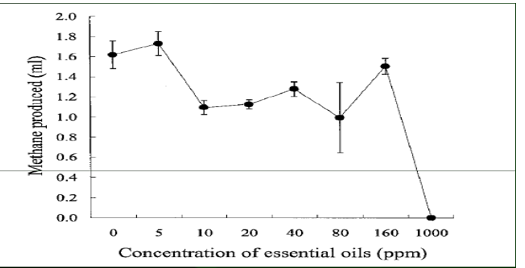
[49] reported that supplementation of coconut with garlic powder (7% + 100 g) could improve in vitro ruminal fluid fermentation in terms of the volatile fatty acid profile, reduced methane losses and reduced protozoa population. [48] reviewed that the effects of the level of dietary lipid on methane emissions in 17 studies and reported that with beef cattle, dairy cows and lambs, there was a proportional reduction of 0.056(g/kg DM intake) in methane for each 10 g/kg DM addition of supplemental fat.
[50] reported that supplementation with Eucalyptus leaf meal at 100 g/d for ruminants could be an alternative feed enhancer: it reduces the production of rumen methane gas in cattle, while the digestibility of nutrients was unchanged. Conversely, [51] reported that increasing the coconut oil and Mago-peellevels decreased proportion of methane production, and that a suitable level should not exceed 6% for coconut oil and 4% DM for MPP supplementation. More over, previous work, based on using plant secondary compounds and oils in both in vitro and in vivotrials, concerning rumen microorganisms, methane production and their impact on the mitigation of methane in the rumen, shows great potential for improving rumen ecology in the study of ruminant productivity.
Table 4: Effect of different plants oils on digestibility and CH4 gas production in various studies.
|
Ingredients |
Level/dosage |
Methane % |
Animal |
References |
|
Garlic powder |
16 mg |
(−) 22.0* |
Buffalo (fluid) |
|
|
Coconut oil |
16 mg |
(+) 6.4* |
Buffalo (fluid) |
|
|
Soapberry fruit and mangosteen peel pellet |
4% |
Holstein heifers |
||
|
Mangosteen peel powder |
100 g/hd/d |
(−) 10.5 |
Beef cattle |
Kongmun P et al.,2009(Wanapat et al., 2009) |
|
Coconut oil |
7% |
(+) 39.5* |
Beef cattle |
Kongmun P et al.,2009 |
|
Coconut oil |
7% |
(−) 10.2* |
Buffalo |
|
|
Coconut oil Garlic powder |
8:4 (mg) |
(−) 18.9* |
Buffalo |
|
|
Coconut oil + Garlic powder |
7% + 100 g |
(−) 9.1* |
Buffalo |
|
|
Eucalyptus oil |
Sheep |
|||
|
Eucalyptus oil |
Buffalo |
|||
|
Eucalyptus meal leaf |
100 g/d |
Reduce |
Cow |
Manh NS,et a.,l 2012 |
Garlic oil and its major components showed CH4 inhibition in batch incubation [53]. [54], also observed a CH4 mitigating effect when adding garlic to the diet of sheep. Another effect is the increase in the propionate-to-acetate ratio resulting in lower amounts of H2 available (reviewed by [33].
Inophores: Monensin (Trade name Rumensin) is the most commonly used ionophore in ruminant nutrition and was originally developed as coccidiostat in poultry [55]. In vivostudies have shown that animals treated with monensin emit reduced levels of CH4 [25,56] but others have reported no significant effect [56] ( Waghorn et al., 2008). Monensin should reduce CH4emissions because it reduces DMI, and because of a shift in rumen VFA proportions towards propionate and a reduction in ruminal protozoa numbers (Singh, 2010). FAO, [27] report that ionophores, through their effect on feed efficiency and reduction in CH4 per unit of feed, would likely have a moderate CH4 mitigating effect in ruminants fed high grain or mixed grain-forage diets. The effect is dose-, feed intake-, and diet composition dependent.
Organic Acids: Organic acids are generally fermented to propionate in the rumen, and in the process reducing equivalents are consumed. Thus they can be an alternative sink for hydrogen and reduce the amount of hydrogen used in CH4 formation. The organic acids such as malate, fumarate, succinate, citrate etc prioponate precursors it has been demonstrated both in vitroand in vivothat their addition to the diet reduce methane production, with the response being dose dependent. Table 5.
Table 5: Feed additives and their presumed mechanism to reduce rumen methanogenesis, specifics need to be considered, supposed reduction potential and recommendations for further research.
|
Group Examples |
Mechanism of CH4 reduction |
Specifics need to Be considered |
Supposed reduction potential In vivo |
Need for further Investigations |
References readings |
|
Lipids Fatty acids Oils
|
Reduced activity of methanogens and protozoa; decreased organic matter fermentation; enhanced propionate production; biohydrogenation of fatty acids |
Total fat should not exceed 6 % of dietary DM |
~25 % |
Long-term effects on methane and composition of microbial community, microbial protein synthesis, fertility |
|
|
Ionophores Monensin |
Inhibition of Gram-positive bacteria and protozoa; enhanced propionate production; lack of substrate for methanogens; improved feed efficiency |
Banned in the EU; microbes may |
~ 30 % |
Long-term effects on methane and composition of microbial community, microbial protein synthesis |
|
|
Secondary plant compounds Tannins Saponins Essential oils |
Antimicrobial activity; reduced hydrogen availability |
High variation; optimum dose unknown; may affect digestibility |
~ 29 % |
Long-term effects on methane and composition of microbial community, microbial protein synthesis, comparison between in vitro and in vivo, use of more defined substances |
(Beauchemin et al., 2007);(Carulla et al., 2005b); Hook et al., 2010; |
|
Organic acids Fumarate Malate |
Alternative hydrogen sink; enhanced propionate production |
May affect Digestibility |
~ 10 % |
Long-term effects on methane and composition of microbial community. |
(Aluwong et al., 2011);(Clark et al., 2011); Hook et al., 2010 |
Development of pelleted feeds: Pellet products such as Mago-pel (mangosteen peel pellet), Maga-lic (mangosteen peel with garlic powder pellet), Maga-ulic (mangosteen peel pellet with urea and garlic powder), LLP (leucaena leaf pellet), MUP (mulberry leaf pellets) and SWEPP (sweet potato vine pellet with 10% urea) have report that can decrease CH4 emission by improving nutrient digestibility and rumen fermentation [52]. See Table 6
Table 6: Feed ingredients and chemical composition of Mago-pel, Maga-lic, Maga-ulic, LLP, MUP and SWEPP
|
Items |
Mago-Pel |
Maga- lic |
Maga-ulic |
LLP |
MUP |
SWEPP |
|
Ingredient |
% of Dry Matter |
|||||
|
Mangosteen peel powder |
98.5 | 93.5 | 91.5 |
- |
- |
- |
|
Garlic powder |
- |
5 |
5 |
- |
- |
- |
|
Leucaena leaf meal |
- |
- |
- |
81 |
- |
- |
|
Mulberry meal |
- |
- |
- |
- |
82 |
- |
|
Sweet potato vine |
- |
- |
- |
- |
- |
81.5 |
|
Cassava starch |
0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
|
Urea |
- |
- |
10 |
10 |
10 |
|
|
Molasses |
1 |
1 |
1 |
5 |
4.5 |
5 |
|
Mineral mixture |
- |
- |
- |
1 |
1 |
1 |
|
Salt |
- |
- |
- |
1 |
1 |
1 |
|
Chemical composition |
- |
- |
- |
1 |
1 |
1 |
|
Dry matter |
93.3 | 93.1 | 92.7 | 92.9 | 92.3 | 95.6 |
|
|
% of Dry Matter |
|
||||
|
Organic matter |
96.5 | 96.4 | 96.5 | 91.3 | 88.2 | 81.4 |
|
Crude protein |
21.2 | 21.5 | 22.1 | 42.2 | 48.7 | 40.5 |
|
Neutral detergent fiber |
57.3 | 57.2 |
57 |
44 |
20.4 | 33.1 |
|
Acid detergent fiber |
48.6 | 48.2 | 48.3 |
20 |
14.5 | 27.8 |
and Figure 1. Steps of preparation. Manasriet al. (2012) reported that supplementation with Maga-lic at200 g/hd/d improved ruminal fermentation, especially increasing the proportion of propionate and reducing methane gas production in beef cattle steers. In addition, the acetate content, the acetate: propionate ratio, the protozoa population and methane production were all reduced, whereas the propionate production and bacterial population increased in the pellet-supplemented group and were highest in the Maga-ulic-supplemented treatment. Table 7and Figure 3.
Table 7: Effect of of Mago-pel, Maga-lic, Maga-ulic, LLP, MUP, SWEPP on DMI, digestibility, rumen volatile fatty acid (VFA) production and ruminal microorganisms
|
Pelleting |
suppl |
Animal |
DMI |
VFA |
CH4 |
MPS |
References |
||||
|
C2 |
C3 |
C4 |
|||||||||
|
MUP |
600 g/hd/d |
Buffalo |
↑ |
↑ |
↓ |
↑ |
↑ |
↓ |
Nd |
↓ |
|
|
MUP |
600 g/hd/d |
Buffalo |
↑ |
nd |
nd |
nd |
nd |
Nd |
↑ |
Nd |
|
|
Mago-Pel |
300 g/hd/d |
Dairy cow |
Nc |
nc |
nc |
nc |
nc |
Nc |
↑ |
↓ |
|
|
Maga- lic |
200 g/hd/d |
Dairy cow |
Nc |
↑ |
↓ |
↑ |
nc |
↓ |
Nd |
↓ |
|
|
Maga-ulic |
200 g/hd/d |
Dairy cow |
Nc |
↑ |
↓ |
↑ |
nc |
↓ |
↑ |
↓ |
|
|
LLP |
450 g/hd/d |
Buffalo |
↑ |
nd |
nd |
nd |
nd |
Nd |
↑ |
↓ |
|
Abbreviations:MUP= mulberry leaf pellet, Mago-pel mangosteen peel pellet, Maga-lic mangosteen peel and garlic pellet, Maga-ulic mangosteen peel, garlic and urea pellet, LLP =leucaena leaf pellet, VFA= volatile fatty acid, C2= acetic acid, C3 =propionic acid, C4= butyric acid, CH4 methane production, increase (↑), decrease (↓) from control group, nd =not determined, nc =no change. Source:[52]
Figure 3: Processing chart for pelleting the products (Mago-pel, Maga-lic, Maga-ulic, LLP, MUP and SWEPP).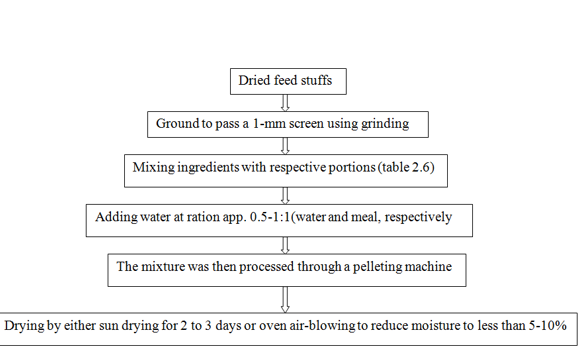
Table 8: Effect of mangosteen peel supplementation on rumen volatile fatty acid, dry matter intake, digestibility and methane production in ruminants using in vitroand in vivostudies.
|
Substrate |
Level |
Species |
TVFA |
DMI |
CH4 |
References |
|
|
In vivo |
|||||||
|
MP |
200 mg |
Steer |
+ |
|
|
|
|
|
In vitro |
|||||||
|
MP |
100 g/hd/d |
Beef cattle |
+ |
+ |
+ |
- |
|
|
MP |
200 g/hd/d |
Dairy cows |
+ |
nc |
+ |
- |
|
|
MP |
100 g/hd/d |
Native cattle |
+ |
nc |
+ |
- |
|
|
MP |
30 g/kg |
Buffalo |
+ |
nc |
- |
- |
|
|
MPP |
200 g/hd/d |
Beef cattle |
+ |
nc |
+ |
- |
|
|
MPP |
300 g/hd/d |
Dairy cow |
+ |
+ |
nc |
- |
|
|
Co |
|
|
|
|
|
|
|
|
Co+MP |
50 + 30 g/kg |
Buffalo |
- |
nc |
+ |
- |
|
|
MP+GP |
9 + 1% |
Beef cattle |
+ |
nc |
+ |
- |
|
|
MP+GP |
200 g/hd/d |
Beef cattle |
+ |
nc |
+ |
- |
|
Abbreviations:GP= garlic powder, MP= mangosteen peel powder, MPP= mangosteen peel pellet, CO= coconut oil, Nc= not changed. (+) increased, (-) decreased. Source:[52]
Table 8 presents the data from both in vitro and in vivo trials using mangosteen peel powder (MP) with or without other sources on rumen fermentation. Based on these results, MP supplementation both for in vitro and in vivo trials significantly increased the production of total volatile fatty acids (P < 0.05), as well as propionate production, while acetate, butyrate production and the acetate: propionate ratio were significantly decreased (P < 0.05). Condensed tannins (CT) and saponins contained in MP could contribute to the above effects. Similar effects, especially regarding the acetate: propionate ratio, were found by [25] while total volatile fatty acids were decreased. The effects of supplementation with MP on DM intake, digestibility and rumen methane production are reported in Table 8. These findings showed that MP supplementation did not affect DM intakes, while digestibility and rumen methane production (by estimation using volatile fatty acid concentration) were significantly decreased (P <0.05).
Manure management systems (MMS)
Manure management refers to manure accumulation and collection in buildings, storage, processing and application to crops. It is well known that GHG emissions (mainly CH4 and N2O) from manure differ significantly depending on the management system employed to process them. Therefore, strategies for mitigating net GHG emissions should be aimed to manipulate manure properties or the conditions under which CH4 and N2O are produced and utilized during manure storage and treatment. However, GHG mitigation options are critical and depend on several factors. These factors are economic, technical and material resources, climatic conditions, existing manure management practices, bio-energy sources, and a source of high conditions, existing manure management practices, bio-energy sources, and a source of high quality fertilizer and soil amendments. One such approach is to manipulate livestock diet composition and/or include feed additives to alter manure pH, concentration and solubility of carbon and nitrogen, and other properties that are pertinent to CH4 and N2O emissions [57].
Nitrogen excreted in urine is predominant in the form of urea that can easily be converted into ammonia and carbon dioxide by the enzyme urease (which is present infeces), thus resulting in emission of ammonia. Nitrogen excreted in feces is mainly present as protein, which is less susceptible to decomposition into ammonia [58]. Therefore, feed management aims at either reducing the nitrogen excretion in feces and urine by matchingthe amount and composition of feed more closely to animal requirements at variousproduction stages, or shifting nitrogen excretion from urine to feces by increasing fibrous feed stuffs in the diet [58]. The use of these strategies can reduce the ammonia emission both for pigs [59] (Kimib et al., 2004) poultry [60,61] and dairy cattle [56]. About 50% of ammoniaemissions to the environment were reduced through feed management for pigs and poultrywhen compared to standard feed composition. However, feed manipulation for ammoniaabatement may negatively affect the emission of methane and nitrous oxide during storageand after land application of the manure [62]. Another manure management option is to change the material used for bedding theanimals, which could also affect manure pH and soluble C and N levels and thus, theemissions during manure storage and treatment. Composting technology, control ofaeration, use of amendments, or co-composting livestock manure with other organic wastecould also potentially modify conditions for GHG production and emission. The use ofcovers may also help retain N nutrients during storage. Floating covers of natural andsynthetic, origin or composites of both have shown substantial reduction in NH3 and H2Semissions when compared with uncover liquid manure. However, little is known about theeffect of covers on GHG emissions. In a two week study, covers generally increased CO2 andCH4 emissions [63].
The amount of CH4 emitted during storage depends on the management system, mainly on storage duration, moisture content, storage temperature, and percentage of anaerobically decomposed manure [11,14]. Dry systems include solid storage, dry feedlot, deep pit stacks and daily spread of the manure. In addition un-managed manure from animals on pasture falls in to this category. Liquid management systems use water to facilitate manure handle. These liquid/slurry systems use concrete tanks and/or lagoons to stored flashed and scraped manure. (EPA, 2008) Liquid management systems often use water to facilitate manure handling. These systems include tanks and lagoons which store manure until it is applied to cropland. Liquid systems create the ideal anaerobic environment for methane production. With the use of liquid-based livestock facilities, the primary method for reducing emissions is to recover the methane before it is emitted into the air. [5].
Manure storage, separation and cover: Greenhouse gas emissions from stored manure are primarily in the form of CH4 (due to anaerobic conditions). Increasing the time of manure storage increases the period during which CH4 (and potentially N2O) is emitted, as well as the emission rate, creating a compound effect (Philippe et al., 2007).One simple way to avoid cumulative GHG emissions is to reduce the time manure is stored (Philippe et al., 2007; Costa et al., 2012). Sommer et al. (2009) simulated several manure management scenarios using data from four European countries and suggested that solids and liquid separation followed by destroy of the solids can reduce overall GHG emissions by 49 to as much as 82 percent compared with the reference system. Several types of manure storage covers have been reported in the literature, ranging from natural crusts in manure storages with high solids content Misselbrook et al. (2005b); and Smith et al. (2007b)., to straw, wood chips, oil layers, expanded clay, wood, semi-permeable and sealed plastic covers Clemens et al. (2006); Guarino et al. (2006); and VanderZaag et al. [63] ( 2009, 2010).The effectiveness of the manure storage cover depends on many factors, including permeability, cover thickness, degradability, porosity and management. Semi-permeable covers such as naturally crusted manures, straw, wood chips and expanded clay generally reduce odour and NH3 and CH4 emissions, with the level of reduction depending on the permeability and thickness of the cover layer. [64] and(Chadwick et al., 2011) conducted studies which showed that additional straw has the potential to reduce GHG emissions during solid manure storage. [64] demonstrated that the mixing of 50% by volume more chopped straw could reduce N2O emissions by 32% from small scale stores of conventional cattle manure. The authors attributed this response to a higher initial C:N ratio (19 compared to 14) and dry matter (DM) content (41% compared to30%) as a result of straw addition. Semi-permeable covers are valuable for reducing NH3, CH4, and odour emissions but likely increase N2O emissions [63] (Sommer et al., 2000; Guarino et al., 2006). Therefore the effectiveness of semi-permeable manure storage covers is not clear, and results vary widely depending on the material and the particular conditions in which it is applied. Covering manure storages with impermeable covers is an effective mitigation practice if the CH4 captured under the cover is burned using a flare system or engine-generator to produce electricity; otherwise the captured CH4 would build pressure inside the storage creating an explosion hazard and/or escape through leaks and cover ruptures. Sealing the manure storage with an impermeable cover results in increased air pressure inside the storage structure reducing the fraction of gases in the gas phase and increasing the fraction trapped in liquid manure. The increased fraction of gases trapped in the liquid fraction of the manure is then released when the pressure in the manure storage container is reducedas manure is transported and applied in the field.
Capturing the gases produced using impermeable membranes, such as oil layers and sealed plastic covers, would result in reduced NH3, N2O and CH4 emissions32. The results from Guarino et al. (2006) and VandeerZaag et al. [63] suggest that using a vegetable oil layer as a manure storage cover, although very effective, is not very practical because of degradability, generation of foul odours and difficulty in preventing the oil film frombecoming mixed or “broken” over the manure surface. Therefore, Impermeable membranes, such as oil layers and sealed plastic covers, are effective in reducing gaseous emissions but are not very practical. Combusting CH4 accumulated under impermeable covers to produce electricity or heat is recommended.
Anaerobic Digester (AD) (Biogasification): AD can reduce GHG emissions related to manure management by more than 50%, mostly in the form of CH4 during storage [65]. When producing electricity through AD, GHG emissions can be further reduced by replacing on-farm fossil fuel-based processes [66]. Anaerobic digester (AD) the simplest form of recovery system, and can be used at dairy or swine farms in temperate or warm climates. Manure solids are washed out of the livestock housing facilities with large quantities of water, and the resulting slurry flows into an anaerobic primary lagoon. The average retention time for the manure in the lagoon is about 60 days. The anaerobic conditions result in significant methane emissions, particularly in warm climates. The covered lagoons are air-tight and provide the anaerobic conditions under which methane is produced and recovered which can be used as energy [5]. Additionally, during anaerobic digestion of the waste/manure, N2O emission is negligible since N2O is formed during aerobic nitrification and anaerobic denitrification [5]. Also [5] reviewed Saggar S, et al., (2004), this is an important N2O mitigation option which reduce N2O emission in the farming system as follows (1) reduce the total amount of excreta N returned to pasture; (2) increase the efficiency of excreta and/or fertilizer N; and (3) avoid soil conditions that favor N2O emissions. Alternatively, frequent turning can be used to reduce anaerobic zones in the heap. This technique reduced CH4 emissions to about 0.5% of initial C content [65].
Land application: GHG emissions from animal manure and wastewater management systems are influenced by different physicochemical and biological factors. The key factors responsible for CH4, CO2, and N2O emissions are soil moisture, temperature, and manure loading rates by the animal, depth of manure in the pen, redox potential, available C, diets, and microbial process [5]. Temperature is a critical factor regulating processes leading to NH3 (Sommer et al., 2006) and CH4 (Steed and Hashimoto, 1994) emissions from stored manure. Decreasing manure temperature to < 10 °C, by removing the manure from the building and storing it outside in cold climates, can mitigate CH4 emissions [56]. According to FAO, [14] cited in the Clemens et al. (2006); and Amon et al. [65]choosing the right timing and form of application, e.g. subsurface application of manures by injection or drilling at times when crop or grass land N demands are high, will increase plant N use efficiency and limit N2O losses to the environment [67-72].
Composting:Composting is an exotermic, aerobic process of microbial decomposition of organic matter that has several benefits related to manure handling, odour control, manure moisture and pathogen control, OM stabilization, additional farm income, etc. Composted manure solids (following manure separation into solids and liquid) is also being used as bedding in some dairy production systems to reduce cost of production and provide cow comfort, assuming udder health is not compromised [73-77] (Husfeldt et al., 2012). However, due to the nature of the composting process, N losses can be high and are influenced by a number of factors, including temperature, C/N ratio, pH, moisture and material consistency (Zeman et al., 2002).
Other manure treatments: There are many waste treatment systems that are used in processing of human wastes. few of these technologies are used practically for treatment of livestock wastes [78-81]. There are many waste treatment systems that are used in processing of human wastes. Few of these technologies are used practically for treatment of livestock wastes. Several studies have reported treatments other than those reported in sections above. Two biological treatments have been demonstrated to reduce emissions [82-84]. In a laboratory study, Luth et al. (2011) demonstrated that earthworm inclusion in a vermifilter fed with swine manure provided a CH4 sink and decreased emissions of NH3 and N2O emissions [85-88]. Fukumoto et al. (2006, 2010) demonstrated that the addition of nitrite-oxidizing bacteria to swine manure reduced N2O emissions up to 80 percent.
Conclusion
Mitigation is any practice that reduces the net amount of greenhouse gases released into the atmosphere. Improving forage quality and the overall efficiency of dietary nutrient use is an effective way of decreasing GHG emissions per unit of animal product. Using feeds containing plant secondary compounds feed additives such as saponins, tannins, essential oils, development of Pellet products such as Mago-pel (mangosteen peel pellet), Maga-lic (mangosteen peel with garlic powder pellet), Maga-ulic (mangosteen peel pellet with urea and garlic powder), LLP (leucaena leaf pellet), MUP (mulberry leaf pellets) and SWEPP (sweet potato vine pellet with 10% urea) and many other metabolites are use of reducing rumen greenhouse gas especially methane products. Mitigation of GHG emissions from animal waste must be addressed in the context of integrated waste management. Semi-permeable covers are valuable for reducing NH3, CH4 and odour emissions but likely increase N2O emissions; therefore, their effectiveness is not clear and results may vary widely. Impermeable membranes, such as oil layers and sealed plastic covers, are effective in reducing gaseous emissions but are not very practical. Manure as a biomass goes through different chemical and biological processes for bioenergy recovery and thus, reduced methane emission. Anaerobic bio-digesters, covered lagoons or manure storages with methane flaring systems or small electricity generators are gaining popularity as viable technologies to abate GHG emissions from manure storage. In addition, since methane is generated under anaerobic conditions, switching manure management from liquid to dry manure, such as pack-bedded dairy option and hoop structure swine buildings with bedding, are other possibly effective management strategies to reduce methane emission.
Recommendation
Mitigation of GHG emissions from livestocks must be addressed in the context of integrated with animal dietary manipulation and manure waste management. Using feeds containing plant secondary compounds feed additives such as saponins, tannins, essential oils, development of Pellet products such as Mago-pel (mangosteen peel pellet), Maga-lic (mangosteen peel with garlic powder pellet), Maga-ulic (mangosteen peel pellet with urea and garlic powder), LLP (leucaena leaf pellet), MUP (mulberry leaf pellets) and SWEPP (sweet potato vine pellet with 10% urea) and many other metabolites are recommended as a means for reducing rumen greenhouse gas especially methane products. Overall, improving forage quality and the overall efficiency of dietary nutrient use is an effective way of decreasing GHG emissions per unit of animal product. Use of Anaerobic digester is a recommended GHG mitigation strategy that has a significant potential to capture and destroy most CH4 from manure, generates renewable energy and provides sanitation opportunities in developing countries.Anaerobic digestersystems are not recommended for geographic locations with average temperatures below 15°C without supplemental heat and temperature control.Capturing the gases produced from manure using impermeable membranes, such as oil layers and sealed plastic covers and Combusting CH4 accumulated under impermeable covers to produce electricity or heat is recommended.
Finally, further study need to be both conventional and non-conventional feed resources need to be studied their potential to affect greenhouse gas emission by the animals. In the future, comprehensive research into the individual components of essential oils, the physiological status of animals, the nutrient composition of diets and their effects on the rumen microbial ecosystem, methane gas inhibition and metabolism of essential oils will be required to obtain consistent beneficial effects. Manure GHG emission mitigation impermeable membranessuch as oil layers and sealed plastic covers, Anaerobic digester (bio gasification) and the time of manure storage, aeration, slatted floors and stacking,practices should be evaluated further for co benefits & pollution swapping effects at a whole farm level.
References
- Alexandratos N, Bruinsma J (2012) World agriculture towards 2030/2050: the 2012 revision. ESA Working paper Rome, FAO.
- Pelletier N, Tyedmers P (2010) Forecasting potential global environmental costs of livestock production 2000–2050. Proceedings of the National Academy of Sciences 107: 18371-18374.
- Bouwman L, Goldewijk Kk, Van Der Hoek Kw, Beusen Ah, Van Vuuren Dp, et al. (2013) Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proceedings of the National Academy of Sciences 110: 20882-20887.
- Bernstein L, Bosch P, Canziani O, Chen Z, Christ R, et al. (2008) IPCC, 2007: climate change 2007: synthesis report, IPCC.
- Borhan Ms, Mukhtar S, Capareda S, Rahman S (2012) Greenhouse gas emissions from housing and manure management systems at confined livestock operations. Waste Management-An Integrated Vision. InTech.
- Herrero M, Gerber P, Vellinga T, Garnett T, Leip A, et al. (2011) Livestock and greenhouse gas emissions: The importance of getting the numbers right. Animal Feed Science and Technology 166: 779-782.
- Gerber Pj, Steinfeld H, Henderson B, Mottet A, Opio C, et al. (2013) Tackling climate change through livestock: a global assessment of emissions and mitigation opportunities, Food and Agriculture Organization of the United Nations (FAO).
- Naqvi S, Sejian V (2011) Global climate change: role of livestock. Asian Journal of Agricultural Sciences 3: 19-25.
- Alemayehu N (2013) Assessment of environmental-livestock interactions in crop-livestock systems of central Ethiopian highlands. University of South Africa.
- Pelletier N, Pirog R, Rasmussen R (2010) Comparative life cycle environmental impacts of threebeef production strategies in the Upper Midwestern United States. Agric. Syst. 103: 380-389.
- Steinfeld H, Gerber P, Wassenaar T, Castel V, Rosales M, et al. (2006) Livestock’s long shadow. Environmental Issues and Options, FAO, Rome.
- Stokstad E (2014) Ammonia pollution from farming may exact hefty health costs. Science 343: 238-238.
- Eshel G, Shepon A, Makov T, Milo R (2015) Partitioning United States' feed consumption among livestock categories for improved environmental cost assessments. The Journal of Agricultural Science 153: 432-445.
- Solomon S (2007) Climate change 2007-the physical science basis: Working group I contribution to the fourth assessment report of the IPCC, Cambridge University Press.
- Hou Y, Velthof Gl, Oenema O (2015) Mitigation of ammonia, nitrous oxide and methane emissions from manure management chains: a meta-analysis and integrated assessment. Global change biology 21: 1293-1312.
- Clarisse L, Clerbaux C, Dentener F, Hurtmans D, Coheur Pf, et al. (2009) Global ammonia distribution derived from infrared satellite observations. Nature Geoscience 2: 479-483.
- Davidson Ea (2009) The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nature Geoscience2: 659-662.
- Sejian V, Lal R, Lakritz J, Ezeji T (2011) Measurement and prediction of enteric methane emission. International Journal of Biometeorology 55: 1-16.
- Smithson Pa (2002) IPCC, 2001: climate change 2001: the scientific basis. Contribution of Working Group 1 to the Third Assessment Report of the Intergovernmental Panel on Climate Change, edited by JT Houghton, Y. Ding, DJ Griggs, M. Noguer, PJ van der Linden, X. Dai, K. Maskell and CA Johnson (eds). Cambridge University Press, Cambridge, UK, and New York, USA, 2001. No. of pages: 881. Price£ 34.95, US $49.95, ISBN 0-521-01495-6 (paperback).£ 90.00, US $130.00, ISBN 0-521-80767-0 (hardback). International Journal of Climatology 22: 1144-1144.
- Hristov A, Hanigan M, Cole A, Todd R, Mcallister T, et al. (2011) Review: ammonia emissions from dairy farms and beef feedlots 1. Canadian journal of animal science 91: 1-35.
- Agrawal D, Kamra D (2010) Global warming: Role of livestock and mitigation strategies. International conference on physiological capacity building in livestock under changing climate scenario of SAPI (Society of Animal Physiologist of India), Indian Veterinary Research Institute (IVRI), Izatnagar. 11-13.
- Sejian V, Naqvi S (2012a) Livestock and climate change: mitigation strategies to reduce methane production, INTECH Open Access Publisher.
- Blaxter KL, Clapperton JL (1965) Prediction of the amount of methane produced by ruminants. Br J Nutr 19: 511-522. [crossref]
- Johnson KA, Johnson DE (1995) Methane emissions from cattle. J Anim Sci73: 2483-2492. [crossref]
- Beauchemin K, Mcginn S (2005) Methane emissions from feedlot cattle fed barley or corn diets. Journal of animal science83: 653-661.
- Beauchemin K, Kreuzer M, O’mara F, Mcallister T (2008) Nutritional management for enteric methane abatement: a review. Animal Production Science48: 21-27.
- Hristov An, Oh J, Lee C, Meinen R, Montes F, et al. (2013) Mitigation of greenhouse gas emissions in livestock production – A review of technical options for non-CO2 emissions.Edited by Pierre J. Gerber, Benjamin Henderson and Harinder P.S. Makkar. FAO Animal Production and Health Paper No. 177. FAO, Rome, Italy
- Beauchemin Ka, Janzen Hh, Little Sm, Mcallister Ta, Mcginn Sm, et al. (2011) Mitigation of greenhouse gas emissions from beef production in western Canada – Evaluation using farm-based life cycle assessment. Anim. Feed Sci. Technol. 166-167: 663-677.
- Mcallister T, Cheng Kj, Okine E, Mathison G (1996) Dietary, environmental and microbiological aspects of methane production in ruminants. Canadian journal of animal science 76: 231-243.
- Ørskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, et al. (1986) Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 119: 1467-1475.
- Bodas R, Prieto N, García-González R, Andrés S, Giráldez FJ, et al. (2012) Manipulation of rumen fermentation and methane production with plant secondary metabolites. Animal Feed Science and Technology 176: 78-93.
- Patra, Saxena J (2009) The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutrition research reviews, 22: 204-219.
- Patra Ak, Saxena J (2010) A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry 71: 1198-1222.
- Carulla J, Kreuzer M, Machmüller A, Hess H (2005a) Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Crop and Pasture Science56: 961-970.
- Grainger C, Clarke T, Auldist M, Beauchemin K, Mcginn S, et al. (2009) Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Canadian journal of animal science89: 241-251.
- Hess H, Monsalve L, Lascano C, Carulla J, Diaz T, et al. (2003) Supplementation of a tropical grass diet with forage legumes and Sapindus saponaria fruits: effects on in vitro ruminal nitrogen turnover and methanogenesis. Crop and Pasture Science54: 703-713.
- Jayanegara A, Wina E, Soliva C, Marquardt S, Kreuzer M, et al. (2011) Dependence of forage quality and methanogenic potential of tropical plants on their phenolic fractions as determined by principal component analysis. Animal Feed Science and Technology 163: 231-243.
- Bhatta R, Uyeno Y, Tajima K, Takenaka A, Yabumoto Y, et al. (2009) Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. Journal of Dairy Science92: 5512-5522.
- Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in ecological research 30: 1-67.
- Patra A, Kamra D, Agarwal N (2006) Effect of plant extracts on in vitro methanogenesis, enzyme activities and fermentation of feed in rumen liquor of buffalo. Animal Feed Science and Technology 128: 276-291.
- Hervás G, Frutos P, Giráldez Fj, Mantecón Ár, Del Pino Macá (2003) Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Animal Feed Science and Technology 109: 65-78.
- Beauchemin K, Mcginn S (2006) Methane emissions from beef cattle: effects of fumaric acid, essential oil, and canola oil. Journal of animal science84: 1489-1496.
- Mao H, Wang Jk, Zhou YY, Liu JX (2010) Effects of addition of tea saponins and soybean oil on methane production, fermentation and microbial population in the rumen of growing lambs. Livestock Science 129: 56-62.
- Goel G, Makkar H, Becker K (2008) Changes in microbial community structure, methanogenesis and rumen fermentation in response to saponin-rich fractions from different plant materials. Journal of Applied Microbiology 105: 770-777.
- Santoso B, Mwenya B, Sar C, Gamo Y, Kobayashi T, et al. (2004) Effect of Yucca schidigera with or without nisin on ruminal fermentation and microbial protein synthesis in sheep fed silage-and hay-based diets. Animal Science Journal 75: 525-531.
- Holtshausen L, Chaves A, Beauchemin K, Mcginn S, Mcallister T, et al. (2009) Feeding saponin-containing Yucca schidigera and Quillaja saponaria to decrease enteric methane production in dairy cows. Journal of Dairy Science 92: 2809-2821.
- Mihaliak Ca, Gershenzon J, Croteau R (1991) Lack of rapid monoterpene turnover in rooted plants: implications for theories of plant chemical defense. Oecologia 87: 373-376.
- Benchaar C, Mcallister T, Chouinard P (2008) Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schidigera saponin extracts. Journal of Dairy Science91: 4765-4777.
- Kongmun P, Wanapat M, Pakdee P, Navanukraw C (2010) Effect of coconut oil and garlic powder on in vitro fermentation using gas production technique. Livestock Science 127: 38-44.
- Manh N, Wanapat M, Uriyapongson S, Khejornsart P, Chanthakhoun V, et al. (2012) Effect of eucalyptus (Camaldulensis) leaf meal powder on rumen fermentation characteristics in cattle fed on rice straw. African Journal of Agricultural Research 7: 1997-2003.
- Pilajun R, Wanapat M (2011) Effect of coconut oil and mangosteen peel supplementation on ruminal fermentation, microbial population, and microbial protein synthesis in swamp buffaloes. Livestock Science 141: 148-154.
- Wanapat M, Kang S, Polyorach S (2013) Development of feeding systems and strategies of supplementation to enhance rumen fermentation and ruminant production in the tropics. Journal of animal science and biotechnology 4: 32.
- Busquet M, Calsamiglia S, Ferret A, Carro M, Kamel C, et al. (2005) Effect of garlic oil and four of its compounds on rumen microbial fermentation. Journal of Dairy Science 88: 4393-4404.
- Patra A, Puchala R, Detweiler G, Dawson L, Sahlu T, et al. (2008) Tethering meat goats grazing forage of high nutritive value and low to moderate mass. Asian-Aust. J. Anim. Sci 21: 1252-1261.
- Chapman HD, Jeffers TK, Williams RB (2010) Forty years of monensin for the control of coccidiosis in poultry. Poult Sci89: 1788-1801. [crossref]
- Van Duinkerken G, André G, Smits MC, Monteny GJ, Sebek LB (2005) Effect of rumen-degradable protein balance and forage type on bulk milk urea concentration and emission of ammonia from dairy cow houses. J Dairy Sci 88: 1099-1112. [crossref]
- Sejian V, Naqvi S (2012b) Livestock and climate change: mitigation strategies to reduce methane production. Greenhouse Gases-Capturing, Utilization and Reduction. InTech.
- Melse RW (2009) Air treatment techniques for abatement of emissions from intensive livestock production.
- Cahn TT, Sutton AL, Aarnink AJA, Verstegen MWA, Schrama JW, et al. (1998) Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. Journal Animal Science76: 1887-95.
- Van Middelkoop JH, Van Harn J (1998) The influence of reduced protein levels in broiler feed on NH3 emissions. Transl Silsoe Res Institute66: 34.
- Angel R, Powers W, Applegate T (2008) Diet impacts for mitigating air emissions frompoultry. In: Proceedings of the 8th International Livestock Symposium (ILES VIII), Iguassu Falls, August 31 – September 4.
- Velthof GL, Nelemans JA, Oenema O, Kuikman PJ (2005) Gaseous nitrogen and carbon losses from pig manure derived from different diets. Journal Environmental Quality 34: 698-706.
- Vanderzaag AC, Gordon RJ, Glass VM, Jamiesson RC (2008) Floating covers to reduce gas emissions from liquid manure storage: A review. Transactions of the ASABE 24: 657-671.
- Yamulki S (2006) Effect of straw addition on nitrous oxide and methane emissions from stored farmyard manures. Agriculture, ecosystems & environment 112: 140-145.
- Amon B, Kryvoruchko V, Amon T, Zechmeister-Boltenstern S (2006) Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment. Agriculture, ecosystems & environment112: 153-162.
- Aguirre-Villegas HA, Passos-Fonseca TH, Reinemann DJ, Armentano LE, Wattiaux MA, et al. (2015) Green cheese: Partial life cycle assessment of greenhouse gas emissions and energy intensity of integrated dairy production and bioenergy systems. Journal of Dairy Science 98: 1571-1592.
- Aluwong T, Wuyep P, Allam L (2011) Livestock-environment interactions: Methane emissions from ruminants. African Journal of Biotechnology10: 1265-1269.
- Carulla J, Kreuzer M, Machmüller A, Hess H (2005b) Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Australian journal of agricultural research 56: 961-970.
- Clark H, Kelliher F, Pinares-Patino C (2011) Reducing CH 4 emissions from grazing ruminants in New Zealand: challenges and opportunities. Asian-Australasian Journal of Animal Sciences 24: 295-302.
- Hook SE, Wright ADG, Mcbride BW (2010) Methanogens: methane producers of the rumen and mitigation strategies. Archaea, 2010.
- Hu W, Liu J, Wu Y, Guo Y, Ye J, et al. (2006) Effects of tea saponins on in vitro ruminal fermentation and growth performance in growing Boer goat. Archives of Animal Nutrition60: 89-97.
- Hung L, Wanapat M, Cherdthong A (2013) Effects of leucaena leaf pellet on bacterial diversity and microbial protein synthesis in swamp buffalo fed on rice straw. Livestock Science151: 188-197.
- Huyen N, Wanapat M, Navanukraw C (2012) Effect of mulberry leaf pellet (MUP) supplementation on rumen fermentation and nutrient digestibility in beef cattle fed on rice straw-based diets. Animal Feed Science and Technology 175: 8-15.
- Kim IB, Ferket PR, Powers WJ, Stein HH, Van Kempen Tatg, et al. (2004) Effects of different dietary acidifier sources of calcium and phosphorus on ammonia, methane and odorant emission from growing finishing pigs. Asian-austral as Journal Animal Science 17: 1131-8.
- Kongmuna P, Wanapata M, Nontasob N, Nishidac T, et al. (2009) Effect of phytochemical and coconut oil supplementation on rumen ecology and methane production in ruminants. Sustainable Improvement of Animal Production and Health, 197.
- Kumar R, Kamra D, Agarwal N, Chaudhary L (2009) Effect of eucalyptus (Eucalyptus globulus) oil on in vitro methanogenesis and fermentation of feed with buffalo rumen liquor. Animal Nutrition and Feed Technology 9: 237-243.
- Manasri N, Wanapat M, Navanukraw C (2012) Improving rumen fermentation and feed digestibility in cattle by mangosteen peel and garlic pellet supplementation. Livestock Science 148: 291-295.
- Ngamsaeng A, Wanapat M, Khampa S (2006) Effects of mangosteen peel (Garcinia mangostana) supplementation on rumen ecology, microbial protein synthesis, digestibility and voluntary feed intake in cattle. Pakistan Journal of Nutrition 5: 445-452.
- Norrapoke T, Wanapat M, Wanapat S (2012a) Effects of protein level and mangosteen peel pellets (Mago-pel) in concentrate diets on rumen fermentation and milk production in lactating dairy crossbreds. Asian-Australasian Journal of Animal Sciences 25: 971-979.
- Norrapoke T, Wanapat M, Wanapat S (2012b) Effects of protein level and mangosteen peel pellets (Mago-pel) in concentrate diets on rumen fermentation and milk production in lactating dairy crossbreds. Asian-Australasian Journal of Animal Sciences 25: 971.
- Poungchompu O, Wanapat M, Wachirapakorn C, Wanapat S, Cherdthong A (2009) Manipulation of ruminal fermentation and methane production by dietary saponins and tannins from mangosteen peel and soapberry fruit. Archives of Animal Nutrition 63: 389-400.
- Sallam S, Bueno I, Brigide P, Godoy P, Vitti D, et al. (2009) Efficacy of eucalyptus oil on in vitro ruminal fermentation and methane production. Options Mediterraneennes 85: 267-272.
- Sliwinski B, Kreuzer M, Wettstein Hr, Machmüller A (2002) Rumen fermentation and nitrogen balance of lambs fed diets containing plant extracts rich in tannins and saponins, and associated emissions of nitrogen and methane. Archives of Animal Nutrition 56: 379-392.
- Suchitra K, Wanapat M (2008) Effects of mangosteen (Garcinia mangostana) peel and sunflower and coconut oil supplementation on rumen fermentation, milk yield and milk composition in lactating dairy cows. Livest. Res. Rural Dev20.
- Tan N, Wanapat M, Uriyapongson S, Cherdthong A, Pilajun R, et al. (2012) Enhancing mulberry leaf meal with urea by pelleting to improve rumen fermentation in cattle. Asian-Australasian Journal of Animal Sciences 25: 452-461.
- Trinh T, Wanapat M, Thao T (2012) Effect of mangosteen peel, garlic and urea pellet supplementation on rumen fermentation and microbial protein synthesis of beef cattle. Agric J 7: 95-100.
- Wanapat M, Pilajun R, Kongmun P (2009) Ruminal ecology of swamp buffalo as influenced by dietary sources. Animal Feed Science and Technology 151: 205-214.
- World Bank, Mearns R, Norton A (2010) Social dimensions of climate change. Washington, DC: World Bank.
