Hydrogen Generation and Accumulation in Steel and Graphite Irradiated in Inert Environment
Evgenii Krasikov1*
1 National Research Centre “Kurchatov Institute”, Russia.
*Corresponding Author: Evgenii Krasikov, National Research Centre “Kurchatov Institute”, Russia, TEL: 7499-196-9639 ; FAX: 7499-196-9639;E-mail:ekrasikov@mail.ru
Citation: Evgenii Krasikov (2019) Hydrogen Generation and Accumulation in Steel and Graphite Irradiated in Inert Environment. Nano Technol & Nano Sci J 2:112.
Copyright: © 2019 Evgenii Krasikov, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received date: Sep 26, 2019; Accepted date: Oct 18, 2019; Published date: Oct 23, 2019.
Abstract
In traditional power engineering hydrogen may be one of the firstprimary source of equipment damage. This problem has high actuality for both nuclear and thermonuclear power engineering. Study of radiation-hydrogen embrittlement of the steel raises the question concerning the unknown source of hydrogen in reactors. Later unexpectedly high hydrogen concentrations were detected in irradiated graphite.
It is necessary to look for this source of hydrogen especially because hydrogen flakes were detected in reactor vessels of Belgian NPPs.
As a possible initial hypothesis about the enigmatical source of hydrogen one can propose protons generation during beta-decay of free neutrons поÑкольку inasmuch as protons detected by researchers at nuclear reactors as witness of beta-decay of free neutrons
Keywords
Nuclear Reactor, Steel, Graphite, Source of Hydrogen
Introduction
It is known that in traditional power engineering hydrogen may be one of the first primary source of equipment damage [1]. This problem has high actuality for both nuclear and thermonuclear power engineering [2]. Particularly reactor pressure vessels (RPV) of the WWER-440/230 project was manufactured without stainless cladding that is were in contact with primary circuit water and accessible for hydrogen as a product of RPV wall corrosion. Analysis of the combined radiation-hydrogenation embrittlement of the 48TS type vessel steel was performed in [3] where at the mention of the American [4] and own data question concerning unknown source of hydrogen in metal that was irradiated in nuclear reactor in hermetic ampoules (was named as “irradiation-produced hydrogen” (IPH) was raised.
Materials and Methods
Table 1 lists chemical composition of the RPV steel used (48TS type). A-543 type US steel takes for comparison.
Table 1: Chemical composition of the RPV steels A-543 and 48TS (%%mass)
| Type | C | Si | Mn | P | S | Cu | Cr | Mo | Ni |
|---|---|---|---|---|---|---|---|---|---|
| 48TS | 0,16 | 0,30 | 0,43 | 0,014 | 0,011 | 0,11 | 2,75 | 0,67 | 0,16 |
| Ð543 | 0,14 | 0,18 | 0,20 | 0,011 | 0,015 | 0,07 | 1,60 | 0,50 | 3,01 |
4% solution of H2SO4 was used for additional electrolytic hydrogenation of the specimens (current density 0,1A/cm2).
Hydrogen concentration (C) was determined by thermal degasation method at temperatures up to 1000°C with gas chromatograph (thermal conductivity detector) registration of gas released.
Experimental Results and Discussion
Determination of the hydrogen content in the irradiated steel fulfilled in the USA went to unexpected result: hydrogen content noticeably exceeded the quantity rated at (n,p) transmutation reaction: less than 0,1 ppm.
Results of the IPH concentration in steel analysis carried out in the USA are shown in Table 2 [4]. One can see that the greater the fast neutron fluence (FNF) the greater the hydrogen content.
Table 2: Dependence of the IPH concentration in steel versus FNF (E>1MeV)
| FNF, ×1018Ñm-2; tirr= 225-300°Ð¡. | 0 | 7 | 200 | 400 |
| IPH concentration, ppm; t°degasation=1000°Ð¡. | 0,2 | 0,9 | 1,7 | 2,1 |
Ageing of the steel at 100-325°C during 48 hours revealed that IPH is not diffusible up to irradiation temperature that is IRH are in the irradiation produced traps. Inasmuch as IPH at temperatures of mechanical tests was immovable indicated values were subtracted from total quantity of hydrogen measured.
In I.V. Kurchatov Institute at several experiments was determined that steel specimens irradiated at relatively low (100-140°C) temperatures in sealed Ar contained ampoules hydrogen content was many times higher relatively initial content but was independent on FNF (Table 3) [3].
Table 3: Dependence of the IPH concentration in steel versus FNF (E>0,5MeV)
| FNF, ×1018Ñm-2; tirr= 100-140°Ð¡. | 0 | 100 | 170 | 190 | 270 | 450 | 500 |
| IPH concentration, ppm; Tdegassing=300°Ð¡. | 0,2 | 2,9 | 4,3 | 13,3 | 24,9 | 9,8 | 3,1 |
Degassing kinetics is plotted in Figure 1, 2.
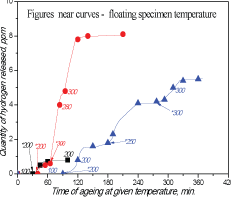
Figure 1: IPH degassing kinetics for irradiated steel (4,5×1020Ñm-2 at 140°Ð¡).
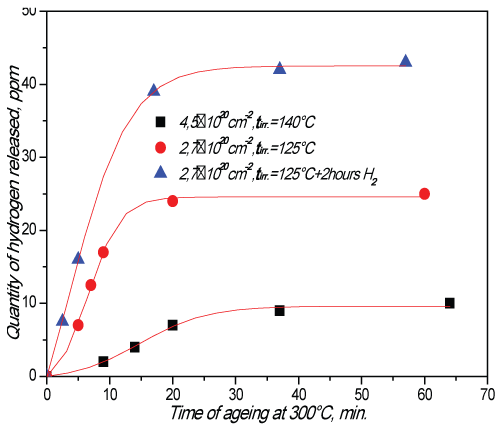
As one can see from Fig.1 that RIH discharge starts when heating temperature exceeds the irradiation temperature. It means that RIH is accumulated in radiation defects (traps).
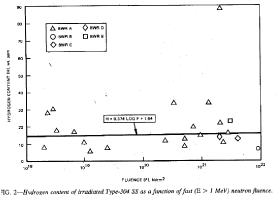
Rather later data appear оn unexpectedly high hydrogen concentrations in stainless steels irradiated in BWR type reactors (Figure 3 [5]) and high generations of hydrogen and helium in nickel [6].
Surprisingly high hydrogen concentrations were revealed in irradiated graphite [7]. Table 4 presents the results of the thermal degasation of the GR-280 type graphite.
Table 4: Results of the thermal degassing of the GR-280 type graphite
| FNF, Ñm-2 (Е>0,5MeV) | tirr, *С | Quantity of hydrogen released, ppm | ||
|---|---|---|---|---|
| tdegassing, *С | Total ppm | |||
| 1000 | 1800 | |||
| 0 | 2,2 1,5 |
4,2 2,9 |
6,4 4,4 |
|
| 1*1022 | 500 | 20,8 49,7 41,2 19,5 |
- - 1342 864 |
20,8 49,7 1383 883 |
| 2*1021 | 1100 | - - |
369 327 |
369 327 |
As it is seen hydrogen concentrations in irradiated specimens is one-two orders of magnitude higher than in unirradiated ones.
It is necessary to look for enigmatic source of hydrogen especially because in frame of inspections numerous flows were detected in the forged rings of the reactor pressure vessels in the Belgian nuclear power plants Doel 3 and Tihange 2 [8,9]. The owner Electrabel claimed that flaws were “most likely” hydrogen flakes.
One of the unobvious but probable initial hypothesis on enigmatic source of the hydrogen in operating nuclear reactor is generation of protons as a product of beta-decay of free neutrons (lifetime ~15 min.) [10].
References
- Vainman AB (1990) Hydrogen embrittlement of the highpressure vessels. Kiev, Naukova dumka, (In Russian).
- Alekseenko NN (1997) Radiation Damage of Nuclear Power Plant Pressure Vessel Steels. ANS p.97.
- Krasikov EA (1974) Investigation of hydrogen embrittlement and hydrogen diffusion in irradiated steel, Ph.D Thesis, ?oscow (In Russian).
- Brinkmann CR (1970) Effects of Hydrogen on the Ductile Properties of Irradiated Pressure Vessel Steels. Report IN-1359, NRTS, Idaho Falls.
- Jacobs AI Hydrogen buildup in Irradiated Type-304 Stainless Steel. ASTM STP 956. F.A. Garner, and N. Igata, Eds. ASTM, Philadelphia, pp.239-244.
- Greenwood LR, Garner FA, Oliver DM (2004) Surprisingly Large Generation and Retention of Helium and Hydrogen in Pure Nickel. Journal of ASTM International, April, vol.1 ?4. Paper ID JAI11365, pp.529-539.
- Biriukov AB, Krasikov EA (1998) Impact of neutron irradiation on graphite dehydrogenations. VANT, ser. Thermonuclear fusion ?1-2, p?.3-8. NRC “Kurchatov Institute, Moscow (In Russian).
- Tweer I (2016) Flawed Reactor Pressure Vessels in the Belgian NPPS Doel 3 and Tihange 2 Comments on the FANC Final Evaluation Report 2015.
- ORNL Evaluating of Electrabel Safety Cases for Doel 3/Tihange 2: Final Report (R1). ORNL/TM-2015/59349, Nov. 2015.
- Yu A. Mostoyoi Neutron yesterday, today, tomorrow. Successes of physics (UFN), vol.166, ?9. 1996, pp. 987-1022. (In Russian).
