Pseudo-Proteins: A New Family of Biodegradable Polymers for Sophisticated Biomedical Applications
Sophio Kobauri1, Temur Kantaria1, Nino Kupatadze1, Nazi Kutsiava2, David Tugushi1, Ramaz Katsarava1,2*
1 Institute of Chemistry and Molecular Engineering, Agricultural University of Georgia, Kakha Bendukidze University Campus, # 240 David Aghmashenebeli Alley, 0131 Tbilisi, Georgia.
2 Centre for Medical Biotechnology and Bioengineering, Georgian Technical University, 77, Merab Kostava St, 0160 Tbilisi, Georgia.
*Corresponding Author: Ramaz Katsarava, Institute of Chemistry and Molecular Engineering, Agricultural University of Georgia, Kakha Bendukidze University Campus, # 240 David Aghmashenebeli Alley, 0131 Tbilisi, Georgia, TEL: 925-984-9446 ; FAX: 925-937-6291;E-mail:r.katsarava@agruni.edu.ge
Citation: Sophio Kobauri, Temur Kantaria, Nino Kupatadze, Nazi Kutsiava, David Tugushi, Ramaz Katsarava, et al. (2019) Pseudo-Proteins: A New Family of Biodegradable Polymers for Sophisticated Biomedical Applications. Nano Technol & Nano Sci J 2:109.
Copyright: © 2019 Ramaz Katsarava, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received date: April 30, 2019; Accepted date: May 04, 2019; Published date: May 09, 2019.
Introduction
Biodegradable Polymers
Polymers called as biodegradable are a special class of high-molecular-weight compounds that breaks down after its intended purpose by chemical (hydrolysis, redox cleavage) or biological (bacterial, enzyme catalysed) decomposition process to result in low-molecular-weight debris (therefore they are called also as resorbable polymers). These polymers are found both naturally occurring and synthetically made, and largely consist of ester, amide (peptide), urethane, ether, and anhydride groups (connecting links in the polymeric backbones). Properties and breakdown mechanism of biodegradable polymers (BPs) are determined by their structure. These polymers are often synthesized by condensation reactions or ring opening polymerization. Today there are vast examples and applications of biodegradable polymers in medicine, agriculture, veterinary, food processing, packaging, as eco-friendly materials. In medicine, biodegradable (resorbable) materials are used as implantable devices such as bone screws, bone plates, contraceptive reservoirs, stents, and vascular stent coatings, wound dressings, surgical sutures, staples, drug delivery vehicles, to name a few. BPs are also employed as membranes, ï¬lms and patches for depositing and growing cell, as tissue engineering scaffolds, etc.
BPs have a long history, and since many are natural products, the precise timeline of their discovery and use cannot be accurately traced. One of the first medicinal uses of a biodegradable polymer was the catgut suture, which dates back to at least 100 AD [1]. The first catgut sutures were made from the intestines of sheep, but modern catgut sutures are made from purified collagen extracted from the small intestines of cattle, sheep, or goats [2]. Today many proteins such as collagen, gelatine, albumin, elastin, fibrin, etc. are used as medicated sponges for burns/wounds, mini-pellets and tablets for protein delivery, carriers for constructing artificial vaccines, gel formulation in combination with liposomes for sustained drug delivery, controlling material for transdermal delivery, microspheres for drug delivery, nanoparticles for drug and gene delivery, as a shell to protect and renders the quantum dots available for in vivo applications, to name a few [3,4]. Along with proteins, among naturally occurring BPs should also be mentioned polysaccharides, hyaluronic acid, nucleic acids, bacterial polyesters (poly-β-hydroxybutyrates).
Synthetic biodegradable polymers and plastics have been intensively developed since 70-80s of the last century [5-7]. Due to the huge commercial interest, many different classes of synthetic biodegradable polymers have been introduced in the art, such as polyesters and poly(ortho esters), polyamides and polyurethanes, poly(ester amide)s and poly (ester urethane)s, polyanhydrides, polyphosphazenes, and amino acid-based polymers (synthetic analogues of proteins).
Even though biodegradable polymers have numerous applications, there are properties that tend to be common among them. All biodegradable polymers should be stable and durable enough for use in their particular application, but upon disposal they should easily break down. Other properties of biodegradable polymers that are common among those used for medicinal usages include being:
• non-toxic;
• capable of maintaining good mechanical integrity until degraded;
• capable of controlled rates of degradation.
They should not to elicit the immune response, and the products of degradation also need not to be toxic, carcinogenic, or teratogenic. These are important as biodegradable polymers are used as resorbable surgical materials and devices as well as for drug delivery where it is critical sustained/controlled release the drug into the body over time instead of all at once. Factors controlling the rate of degradation include molecular weight, hydrophobicity, and degree of crystallinity. The degradation rate depends on the location in the body, which influences the environment surrounding the polymer such as pH, enzymes concentration, and amount of water.
Among the naturally occurring BPs the most applicable for biomedical purposes are proteins. The proteins show a high tissue compatibility owing to a high affinity with tissues that is connected with peptide (NH-CO) bonds. Besides, proteins release α-amino acids upon biodegradation which represent a nutritive material and support cells proliferation and ultimately the regeneration of tissues. However, proteins as biomaterial has some limitations such as batch-to-batch variation, risk of disease transmission and immune rejection. The last limitation is connected with molecular architecture of proteins. It is known that α-amino acids (AAs) in a protein molecule have so-called “head-to-tail” orientation as it is depicted in Figure 1, A (assuming that α-amino group is “head” and α-carboxyl groups is “tail”). This natural proteinaceous architecture of macromolecules containing only amide (peptide) groups is easily recognizable by the immune system of the body. This can cause immune incompatibility of the biomaterial with the body tissues.
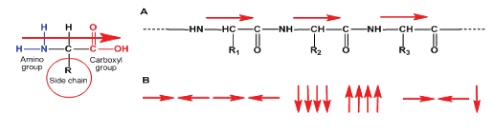
The main advantage of synthetic BPs over the naturally occurring ones consists in low to zero immunogenicity and no risk of disease transmission. However, the most of synthetic BPs listed above do not contain amide (peptide) NH-CO bonds and possess lower compared to the proteins tissue compatibility and, besides, do not release nutritive AAs upon biodegradation. Therefore, more promising from these view-points look AAs-based BPs (AABPs) – man-made analogues of proteins which contain amide NH-CO bonds and release AAs upon biodegradation. These polymers can be designed so to have non-proteinaceous molecular architecture (Figure 2, B) less recognizable by immune system of the organism. Along with amide (peptide) NH-CO bonds AABPs can contain additional hetero-bonds like ester, ether, urea, urethane, etc. in the backbones that can further decrease the immunogenicity of the polymers and, in parallel, to widen the range of their material properties.
The main representatives of synthetic AABBPs introduced in the art since 80s are: poly(amino acid)s (PAAs), pseudo-poly(amino acid)s (PPAAs), poly(depsipeptide)s (PDPs), and pseudo-proteins.
Poly(?-amino acid)s, PAAs
The most rational and commonly used synthesis of PAAs consists in ring-opening polymerization (ROP) of suitable monomers - N-carboxy-α-amino acid anhydrides (NCAs) which readily undergo polymerization with carbon dioxide evolution yielding the corresponding PAAs [8], as it is depicted in Figure 2.
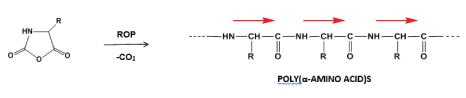
Despite some prospects of the synthetically made PAAs as a potential biomaterials, the studies revealed that most of the PAAs could not be considered as suitable biomedical materials due to their immunogenicity stipulated by proteinaceous molecular architecture [9] along with unfavourable physical-chemical characteristics - with few exceptions, PAAs tend to be insoluble polymers that decompose in the molten state [10,11].
Pseudo-poly(amino acid)s, PPAAs
More promising, compared to PAAs, as biodegradable biomaterials are so called “pseudo-poly(amino acid)s” - the AABPs the backbones of which are constructed by utilizing the side-chain functional groups of suitably modified trifunctional α-L-amino acids or dipeptides made of them [12-14]. Accordingly, pseudo-poly(amino acid)s (PPAAs) have one of the non-proteinaceous molecular architecture (Figure 1,B) and differ from conventional PAAs which have proteinaceous molecular architecture (Figures 1,A and 2). Such an approach offers the opportunity to create polymers from naturally occurring metabolites (AAs) [12-14] but without some of disadvantages of conventional PAAs. Copolymerization of AAs with non-AA monomers has been achieved through a variety of reactions, leading to PPAAs of various classes - polyesters, polyarylates/poly¬carbo¬nates, polyamides, polyureas, polyurethanes, with a wide range of structures and properties. Synthesis of several samples of the PPAAs are given in brief below.
The PPAA of polyester classes (PPAA-PEs) having valuable material properties were synthesized on the basis of hydroxy-AAs such as hydroxyproline and serine.
High-molecular weight PPAA-PEs (MW up to 42 kDa) were obtained by thermal polycondensation of N-substituted trans-4-hydroxy-proline [14-16] as depicted in Figure 3 below:
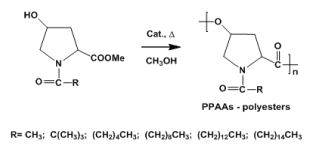
For synthesizing high-molecular-weight (MW up to 30-40 kDa) serine-based PPAA-PEs better best was found the ring-opening polymerization of N-protected-L-serine β-lactone (Figure 4) [13,17].
One of the most promising representatives of the PPAAs are the polymers on the basis of AA tyrosine [14]. The key monomers for synthesizing these PPAAs are tyrosine based bis-phenols such as tyrosine dipeptide and desaminotyrosyl-tyrosine [18-22] (Figure 5).
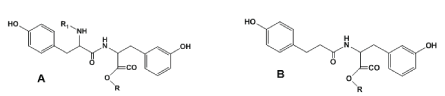
These monomers were used for synthesizing all classes of heterochain polymers available on the basis of bis-phenol type monomers and linked by non-amide bonds such as ester, ether, carbonate or iminocarbonate bonds. Such polymers contain CO-NH bonds in the backbones (which come from the monomers depicted in Figure 5) that provides enhanced affinity and, hence, compatibility of the polymers with tissues [23].
Suitable monomers for synthesizing PPAAs are also three-functional AAs such as α-amino dicarboxylic acids - aspartic and glutamic acids, and diamino α-carboxylic acid lysine which contain side-chain functional groups used for constructing PPAAs (Figure 6).
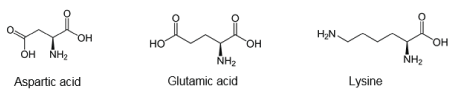
These AAs after suitable modification were used for synthesizing high-molecular-weight polymers. Aspartic and glutamic acids were used for synthesizing polyamides (PAs) [24]. Lysine was used for synthesizing functional PPAAs with free lateral carboxyl groups. On the basis of lysine were obtained PAs [25] and highly functional PAs [26-29], polyureas [30-31] and polyurethanes [32].
Poly(depsipeptide)s, PDPs
Polydepsipeptides (PDPs) represent an important class of AABPs completely composed of naturally occurring (“physiological”) building blocks such as α-amino acids and α-hydroxy acids [33-36]. The most rational way for synthesizing the PDPs is ring-opening polymerization (ROP) of cyclic depsipeptides - morpholine-2,5-diones developed in 1985s [33], as depicted in Figure 7.
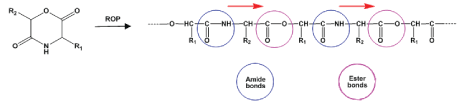
Various PDPs were synthesized by ROP and copolymerization of different morpholine-2,5-dione derivatives including random, alternating, diblock, triblock, functional and graft PDPs [37-44]. It has to be noted that though in the backbones of the PDPs the AAs have “head-to-tail” orientation but they are interleaved with ester bonds that provides non-proteinaceous molecular architecture less recognizable by immune system.
Pseudo-proteins, PPs
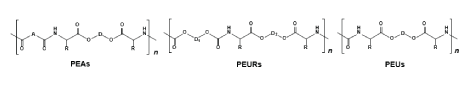
A huge and highly promising family of the synthetic AABPs destined for broad biomedical applications was obtained on the basis of AAs and other non-toxic and vastly available building blocks such as diols and dicarboxylic acids. We used the name pseudo-proteins (PPs) for these polymers to distinguish them from other classes of AABPs discussed above. The first representatives of PPs having valuable material properties were poly(ester amide)s (PEAs) reported in 1994 [45]. Later other classes of PPs such as poly(ester urethanes)s (PEURs) and poly(ester urea)s were reported as well [46,47].
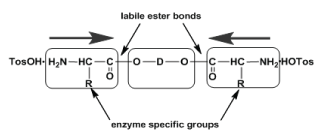
One of the main advantages of the PPs over other classes of the AABPs consist in the key bis-nucleophilic monomers – α,α’-diamine-diesters as stable di-p-toluenesulfonic acid salts (TDADEs). These monomers are basically synthesized in high yields (up to 90-95%) via very simple and cost-effective procedure - direct thermal condensation of two moles of α-amino acids with one mole of diols in refluxed organic solvent (benzene, toluene or cyclohexane) in the presence of two moles (in case of AA arginine four moles) of p-toluenesulfonic acid (Figure 8) which serves as both amino groups protector and the condensation reaction catalyst [45-51].
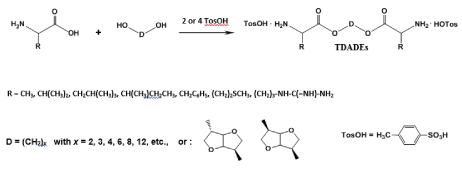
Additional merits of the TDADE-monomers are stability upon storage, highly active terminal amino groups for successful chain growth along with the designed-in non-proteinaceous (“head-to-head”) orientation of amino acids, enzyme specific lateral groups R, and labile (hydrolysable) ester bonds (Figure 9).
The TDADEs are universal bis-nucleophilic monomers which, after interacting with various bis-electrophiles (Figure 10), lead to the synthesis of three basic classes of the PPs such as poly(ester amide)s (PEAs) [45,48,49], poly(ester urethane)s (PEURs) [46,49], and poly(ester urea)s (PEUs) [46,47,49] depicted in Figure 11. Diacid chlorides and activated diesters of diacids are used for synthesizing the PEAs, diol-bis-chloroformates and activated bis-carbonates of diols - PEURs, phosgene/triphosgene and activated carbonates – PEUs.
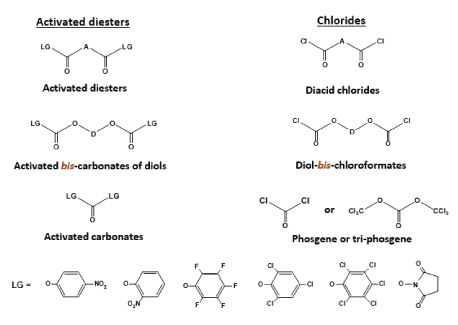
Figure 10: Some key bis-electrophilic monomers for synthesizing PPs. A – a diacid residue, D – a diols residue.
Using the bis-electrophilic monomers above, two techniques of the SGP such as Interfacial Poly-condensation (IP) and Solution Active Polycondensation (SAP) can be applied to the synthesis of the PPs [49].
A variety of the classes and synthetic methods for making thereof allow to tune the structure and material properties of the PPs in the widest range. As expected, based on the non-proteinaceous architecture, PPs show low to zero immunogenicity [35,49,52-58].
The most of PPs are well-soluble in organic solvents such as ethanol and isopropanol (approved for medical applications), N,N-dimethylformamide, tetrahydrofuran, dioxane, chloroform, methylene chloride, and some of them even in acetone and ethylacetate.
The molecular weights of the PPs are reasonably high and vary within 24,000 – 167,000 Da (Mw), polydispersity - within 1.20–1.81. The thermal characteristics of the PPs are also within a wide range: glass transition temperature (Tg) varies within 5 – 102°C and some of the PPs (PEAs and PEUs) are semi-crystalline with melting temperature (Tm) within 103–165°C. The low melting temperatures and solubility of PPs in common organic solvents substantially facilitates their processing into different shapes [48,49].
The chemical structure affects the mechanical properties of PPs, which varies in a wide range: tensile strength from 15-20 (PEURs and some PEAs) to 80-100 MPa (PEUs and some PEAs), elongation at break from 8-100 (PEUs and some PEAs) to 800-1000% (PEURs and some PEAs), Young’s modulus up to 6 GPa (some PEAs and PEUs) [49,59,60]. The PPs can be obtained in form of viscous-flow materials promising for sealing cavities.
The PPs can degrade by both chemical (nonspecific) and enzymatic hydrolysis with a reasonable rates ranging within V = 10-3–10-1 mg/cm2h [48,54,61]. The biodegradation of the PPs proceeds by erosive mechanism without compromising bulk properties that is important when constructing both resorbable surgical devices and therapeutic (drug delivery) systems. The biodegradation rate can be tuned by impregnated or surface immobilized enzymes [61,62]. The biodegradation of PPs starts from the hydrolysis of the ester bonds since they are 103-104 times more active as compared with amide groups. The amide, urethane and urea bonds are hydrolyzed at subsequent stages of biodegradation resulting in ultimate products of biodegradation. The ultimate biodegradation products are: for PEAs – AAs, dicarboxylic acids and diols, for PEURs and PEUs – AAs, diols and carbon dioxide.
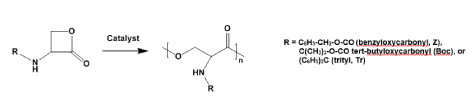
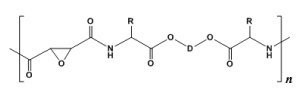
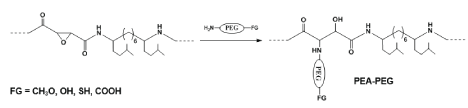
The scopes of applications of PPs can be substantially expanded by designing functional PPs. This can be achieved (i) by combination of TDADEs with functionalized TDADEs or with other types of functional co-monomers, or (ii) by synthesizing various active pre-polymers with subsequent functionalization by means of polymer-analogous reactions. As a result, various types of functionalized PPs were obtained [55]. One of them - epoxy-functionalized PP-PEAs [63] contain active oxirane rings (Figure 12) and be used for covalent attachment of the compounds containing free amino groups.
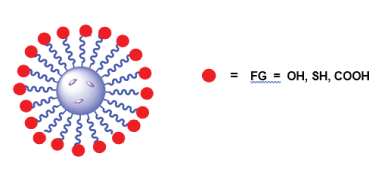
Epoxy-PP-PEAs can further be functionalized by interaction with hetero-bifunctional PEG-derivatives such amine-PEG-carboxymethyl (Figure 13, FG=COOH), amine-PEG-thiol (Figure 13, FG=NH2) or amine-PEG-hydroxyl (Figure 13, FG=OH). After such kind of modification the PPs become water soluble and contain functional groups at attached lateral PEG-substituents remote form the polymeric backbones.
The PP modified with methoxy-PEG-amine (Figure 13, FG = CH3O), labelled as PEA-mPEG represents a kind of biodegradable surfactant which forms micelles in water solution. A comparative study of PEA-mPEG carried out using known surfactants showed very close characteristics of the all surfactants studied (Table 1) in terms of mean particle diameter (MPD), polydispersity index (PDI) and zeta-potential (ZP).
Table 1: Characteristics of micelles of standard surfactants and new biodegradable PP-based surfactant PEA-mPEG from Figure 13 (FG = CH3O).
| Surfactant | MPD (nm) ± SD | PDI ± SD | ZP (mV) ± SD |
|---|---|---|---|
| Tween 20 | 11.3 ± 0.3 | 0.244 ± 0.029 | -9.5 ± 0.8 |
| Brij010 | 19.2 ± 0.3 | 0.173 ± 0.016 | -11.1 ± 0.5 |
| Kolliphor P188 | 9.2 ± 0.4 | 0.373 ± 0.031 | -13.9 ± 1.5 |
| Triton X-100 | 10.4 ± 0.7 | 0.254 ± 0.021 | -6.5 ± 0.5 |
| Mowiol 4-88 | 20.0 ± 1.3 | 0.463 ± 0.039 | -13.0 ± 1.9 |
| PEA-mPEG | 16.2 ±1.0 | 0.174 ± 0.012 | -13.1 ± 1.8 |
± SD – standard deviation of three parallel measurements.
The PEG-modified PP PEA-mPEG is promising as surfactants for fabricating NPs with simultaneous PEGylation of their surface [64]. The PEGylation of NPs which are used as drug delivery vehicles is important to protect them from the attack of immune system of the organism. The PEGylation decreases affinity of plasma proteins (opsonins) for adsorption on NPs - long chains of PEG form a random cloud around the NPs thereby preventing absorption of opsonins and in that way suppressing phagocytosis. Along with the protection of the NPs from phagocytosis the PEGylation substantially increases the bioavailability of NPs [65]. The functional groups of the PEG cloud (functionalized cloud – Figure 14) can be used for the conjugation of various markers, vectors, etc. to the surface of NPs.
A library of cationic PPs was synthesized on the basis of AA arginine. The cationic PPs showed excellent cell compatibility, transfection (important for potential applications in gene therapy), and bactericidal activity [66-68]. The cationic PPs are also suitable for constructing positively charged nanoparticles. A series of positively charged NPs was prepared by blending of regular (neutral) PP-PEA 8L6 and cationic PP-PEA 8R6 (Figure 15). The NPs were fabricated by cost-effective polymer deposition/solvent displacement (nanoprecipitation) method [69]. The zeta-potential of the NPs was changed from -12.5 mV in case of pure 8L6 to +28.0 mV in case of 50/50 blend of 8L6/8R6 (Table 2).
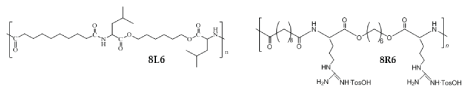
Table 2: The ZP of the NPs made of 8L6/8R6 blends.
| Polymer | Weigh ratio 8L6/8R6 | Zeta-potential (mV)±SD |
|---|---|---|
| 8L6 | 100/0 | –12.5 ±1.9 |
| 8L6 / 8R6 | 95/5 90/10 80/20 70/30 50/50 |
+19.6 ± 3.6 +23.2 ± 4.7 +25.8 ±2.7 +27.2 ±3.2 +28.0 ±4.9 |
NPs were fabricated using Tween 20 as a surfactant
± SD – standard deviation of three parallel measurements.
Positively charged NPs are favourable for penetration through cells and biological barriers - it is known that a positive charge helps with the NPs’ adhesion to the surface of cells and stimulates penetration into the cells via endocytosis [70].
The PPs of PEA and PEU classes were found suitable for fabricating fibrous mats by the electrospinning technique. These mats can be loaded with enzymes and antibacterial drugs including bacteriophages [71-75]. Such loaded fibrous mats are highly promising as soft dressings to treat superficial burns, wounds and ulcers. It is important that the bioresorbable dressing does not stick to the wound and is removed spontaneously after complete healing of the wound/skin.
References
- V Nutton (2012) Ancient medicine (2nd ed.). London: Routledge. ISBN 9780415520942.
- Lippincott, David B. Troy (2005) Remington: The science and practice of pharmacy (21st ed.). Philadelphia, PA: editor. Williams & Wilkins. ISBN 978-0-7817-4673-1.
- V Chak, D Kumar, SVisht (2013) A review on collagen based drug delivery systems Intern. J. Pharm., Teaching & Practices 4: 811.
- D Kumar, RR Kundapur (2015) Editors, Biomedical Applications of Natural Proteins: An Emerging Era in Biomedical Sciences, Springer Briefs in Biochemistry and Molecular Biology.
- R Chandra, Rustgi R (2015) Biodegradable polymers. Progr. Polym. Sci 23: 1273-1335.
- LS Nair, CT Laurencin, (2007) Biodegradable polymers as biomaterials. Progr. Polym. Sci 32: 762-798.
- M Okada (2002) Chemical Synthesis of Biodegradable Polymers, Progr. Polym Sci 27: 87-133.
- M Khuphe, PD Thornton (2018) Poly(amino acids), Chapter 7 in Engineering of Biomaterials for Drug Delivery Systems, ed. A. Parambath, Woodhead Publishing, p.199.
- E Katchalski (1974) Poly(amino acids): achievements and prospects, Wiley, New York.
- A Nathan, J Kohn, Amino Acid Derived Polymers (1994) Chapter 5 in Biomedical Polymers – Designed- to-Degrade Systems, ed. S.W.Shalaby, Hanser Publisher, Munich-Vienna-New York.
- CH Bamford, A Elliott, WE Hanby (1956) Synthetic Polypeptides; Academic Press: New York.
- Kohn J, Langer R (1984) Proceedings of the ACS Division of Polymeric Materials, Science and Engineering; American Chemical Society: Washington, DC, 51, 119.
- QX Zhou, J Kohn (1990) Preparation of Poly(L-serine ester): A Structural Analogue of Conventional Poly(L-serine), Macromolecules 23: 3399.
- J Kohn, R Langer(1987) Polymerization Reactions Involving the Side Chains of a-L-Amino Acids, J. Am. Chem. Soc 109: 817.
- HY Kwon, R Langer (1989) Pseudopoly(amino acids): A Study of the Synthesis and Characterization of Poly(trans-4-hydroxy-N-acyl-L-prolien esters), Macromolecules 22: 3250.
- Yu H (1988) Pseudopoly(Amino Acids): A Study of the Synthesis and Characterization of Polyesters Made from a-L-Amino Acids, Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA.
- I Fietier, A Le Borgne, N Spassky (1990) Synthesis of functional polyesters derived from serine. Polym. Bull. 24: 349.
- J Kohn (1991) Pseudo-poly(amino acids), Drug News Perspect. 4: 289.
- J Kohn (1991) The use of natural metabolites in the design of non-toxic polymers for medical applications, Polym. News 16: 325.
- Pulapura S, Kohn J (1992) Tyrosine-derived polycarbonates: backbone-modified "pseudo"-poly (amino acids) designed for biomedical applications. Biopolymers 32: 411-417. [crossref]
- SI Ertel, J Kohn (1994) Evaluation of a series of tyrosine-derived poly-polycarbonates for biomaterial applications, J. Biomed. Mater. Res. 28: 919.
- SL Bourke, J Kohn (2003) Polymers derived from the amino acid L-tyrosine: polycarbonates, polyarylates and copolymers with poly(ethylene glycol), Adv. Drug Deliv. Rev. 55: 447.
- M Jacoby (2001) Custom–made biomaterials. Chem. Eng. News 79: 30-35.
- R Katsarava, D Kharadze, L Avalishvili (1986) Synthesis of polyamides on the basis of aspartic and glutamic acids, Vysokomolek. Soed. Ser. B 27: 518.
- RD Katsarava, DP Kharadze, NSh Japaridze, TN Omiadze, LM Avalishvili, et al. (1985) Heterochain polymers based on natural amino acids. Synthesis of polyamides from Na,N?-bis(trimethylsilys)lysine alkyl esters. Makromol. Chem. 186: 939-954.
- JJ Bou, S Munoz-Guerra (1995) Synthesis and characterization of a polytartaramide based on L-lysine, Polymer 36: 181-186.
- AC Couffin-Hoarau, M Boustta, M Vert (2001) Enlarging the Library of Poly-(L-lysine citramide) Polyelectrolytic Drug Carriers, J. Polym. Sci.: Part A: Polymer Chem 39: 3475-3484.
- J Huguet, M Boustta, M Vert (1991) Water Soluble Polymers: Synthesis, Solution Properties and Applications; Shalaby in ACS Symposium Series 467; S. W.; McCormick, C. L.; Butler, G. B., Eds.; American Chemical Society: Washington, DC, p 405.
- JL Espartero, B Coutin, H Sekiguchi (1993) Synthesis of a polyamide from L-aspartic acid and L-lysine, Polymer Bulletin 30: 495.
- TN Sentsova, VI Butaeva, YuA Davidovich, SV Rogozhin, VV Korshak, et al. (1977) Synthesis of optically active polyureas composed of natural diaminocarboxylic acids. Dokl. Akad. Nauk SSSR 232: 335-338.
- RD Katsarava, TM Kartvelishvili, YuA Davidovich, SV Rogozhin (1982) New mothod of the synthesis of polyureas by interaction of activated diphenylcarbonates with diamins and their N,N’-bis-tromethylsilyl derivatives, Dokl. Akad. Nauk SSSR 266: 363-336.
- RD Katsarava, TM Kartvelishvili, MM Zaalishvili (1985) Heterochain polymers composed of natural amino acids. Synthesis of new optically active polyurethanes by interaction of Na,N?-bis-carbonyl lysine with diols, Dokl. Akad. Nauk SSSR 281: 591-595.
- J Helder, FE Kohn, S Sato, JW van den Berg, J Feijen, et al. (1985) Synthesis of poly [oxyethylidenecarbonylimino-(2-oxoethylene)] [poly(glycine-D,L-lacatc acid)] by ring opening polymerization, Makromol. Chem. Rapid Commun 6: 9-14.
- A Rodriguez-Galan, L Franco, J Puiggali (2011) Degradable Poly(ester amide)s for Biome¬di¬cal Applications. Polymers 3: 65-99.
- H Sun, F Meng, AA Dias, M Hendriks, J Feijen, et al. (2011) a-Amino Acid Containing Deg¬ra¬¬da¬ble Polymers as Functional Biomaterials: Rational Design, Synthetic Pathway, and Biomedical Applications. Biomacromolecules 12: 1937-1955.
- AC Fonseca, MH Gil, PN Simões (2014) Biodegradable poly(ester amide)s – A remarkable oppo¬rtu¬nity for the biomedical area: Review on the synthesis, characterization and applications. Progr. Polym. Sci 39: 1291-1311.
- PJA In't Veld, PJ Dijkstra, J Feijen (1992) Synthesis of biodegradable polyesteramides with pendant functional-groups J. Makromol. Chem. Phys 193: 2713-2730.
- Feng Y, Lu J, Behl M, Lendlein A (2010) Progress in depsipeptide-based biomaterials. Macromol Biosci 10: 1008-1021. [crossref]
- Y Feng, D Klee, H Höcker (1999) Synthesis and characterization of new ABA triblock copolymers with poly[3(S)-isobutylmorpholine-2,5-dione] and poly(ethylene oxide) blocks. Macromol. Chem. Phys 200: 2276-2283.
- Feng Y, Guo J (2009) Biodegradable polydepsipeptides. Int J Mol Sci 10: 589-615. [crossref]
- T Ouchi, H Miyazaki, H Arimura, F Tasaka, A Hamada, et al. (2002) Synthesis of biodegradable amphiphilic AB-type diblock copolymers of lactide and depsipeptide with pendant reactive groups. J. Polym. Sci. Part A: Polym. Chem 40: 1218-1225.
- K Nagahama, Y Imai, T Nakayama, J Ohmura, T Ouchi, et al. (2009) Thermo-Sensitive Sol-Gel Transition of Poly(depsipeptide-co-lactide)-g-PEG Copolymers in Aqueous Solution. Polymer 50: 3547-3555.
- D Wang, XD Feng (1997) Synthesis of poly (glycolic acid-alt-L-aspartic acid) from a morpholine-2,5-dione derivative. Macromolecules 30: 5688-5692.
- G John, S Tsuda, M Morita (1997) Synthesis and modification of new biodegradable copolymers: Serine/glycolic acid based copolymers. J. Polym. Sci. Part A: Polym. Chem 35: 1901-1907.
- N Arabuli, G Tsitlanadze, L Edilashvili, D Kharadze, T Goguadze, et al. (1994) Heterochain polymers based on natural a-amino acids. Synthesis and enzymatic hydrolysis of regular poly(ester amide)s based on bis(L-phenylalanine) a,? – alkylene diesters and adipic acid. Macromol. Chem. Phys. 195: 2279.
- T Kartvelishvili, G Tsitlanadze, L Edilashvili, N Japaridze, R Katsarava, et al. (1997) Amino acid based bioanalogous polymers. Regular poly(ester urethane)s and poly(ester urea)s based on bis(phenylalanine)-?,?–alkylene diesters. Macromol. Chem. Phys. 198: 1921-1932.
- R Katsarava, D Tugushi, ZD Gomurashvili, Poly (ester urea) Polymers and Methods of Use. US Patent No. 8,765,164.
- R Katsarava, V Beridze, N Arabuli, D Kharadze, CC Chu, et al. (1999) Amino acid based bioanalogous polymers. Synthesis and study of regular poly(ester amide)s based on bis(a-amino acid) ?,?– alkylene diesters and aliphatic dicarboxylic acids, J. Polym. Sci.: Part A: Polym.Chem. 37: 391-407.
- R Katsarava, Z Gomurashvili (2011) Biodegradable Polymers Composed of Naturally Occurring a-Amino Acids in Handbook of Biodegradable Polymers - Isolation, Synthesis, Characterization and Applications, A. Lendlein, and A. Sisson eds., Wiley-VCH, Verlag GmbH & Co. KGaA, p.107.
- Z. Gomurashvili, H. R. Kricheldorf, R. Katsarava, Amino acid based bioanalogous polymers. Synthesis and study of new regular poly(ester amides)s composed of hydrophobic a-amino acids and dianhydrohexitoles. J. Macromol. Sci.-Pure and Appl. Chem. 37, 215-227 (2000).
- M Okada, M Yamada, M Yokoe, K Aoi (2001) Biodegradable Polymers Based on Renewable Resources. V. Synthesis and Biodegradation Behavior of Poly(ester amide)s Composed of 1,4:3,6-Dianhydro-D-glucitol, a-Amino Acid, and Aliphatic Dicarboxylic Acid Units, J. Appl. Polym. Sci 81: 2721-2734.
- M Kropp, KM Morawa, G Mihov, AK Salz, N Harmening, et al. (2014) Biocompatibility of Poly(ester amide) (PEA) Microfibrils in Ocular Tissues, Polymers. 6: 243-260.
- V Andrés-Guerrero, M Zongc, E Ramsay, B Rojas, S Sarkhel, et al. (2015) Novel biodegradable polyesteramide microspheres for controlled drug delivery in Ophthalmology, J. Control. Release. 211: 105-117.
- R Katsarava, N Kulikova, J Puiggalí (2016) Biodegradable Polymers Composed of Amino Acid Based Diamine-Diesters – Promising Materials for the Applications in Regenerative Medicine, J. J. Regener. Med 1: 012.
- N Zavradashvili, G Jokhadze, M Gverdtsiteli, D Tugushi, R Katsarava, et al. (2017) Biodegradable functional polymers composed of naturally occurring amino acids Res. Rev. Polym. 8: 105-128.
- DeFife KM, Grako K, Cruz-Aranda G, Price S, Chantung R, et al. (2009) Poly(ester amide) co-polymers promote blood and tissue compatibility. J Biomater Sci Polym Ed 20: 1495-1511. [crossref]
- M Trollsas, B Maslanka, N Pham, Q Lin, S Hossainy, et al. (2011) Polyesteramide coating for drug eluting stents: controlling drug release by polymer engineering in Active Implants and Scaffolds for Tissue Regeneration. Berlin: Springer; p. 127.
- Y Huang, L Wang, S Li, X Liu, K Lee, et al. (2006) Stent-based tempamine delivery on neointimal formation in a porcine coronary model, Acute Cardiac Care. 8: 210-215.
- KS Stakleff, F Lin, LA Smith Callahan, MB Wade, A Esterle, et al. (2013) Resorbable, amino acid-based poly(ester urea)s crosslinked with osteogenic growth peptide with enhanced mechanical properties and bioactivity, Acta Biomater 9: 5132-5142.
- G Policastro, F Lin, M Becker, KS Stakleff, A Esterle, et al. (2015) OGP Functionalized Phenylalanine-based Poly(ester urea) for Enhancing Osteoinductive Potential of human Mesenchymal Stem Cells, 249th ACS National Meeting & Exposition, Denver, CO, USA, March 22-26, 93, 45.
- Tsitlanadze G, Machaidze M, Kviria T, Djavakhishvili N, Chu CC, et al. (2004) Biodegradation of amino-acid-based poly(ester amide)s: in vitro weight loss and preliminary in vivo studies. J Biomater Sci Polym Ed 15: 1-24. [crossref]
- GTsitlanadze, T Kviria, CC Chu, R Katsarava (2004) Biodegradation of amino acid based poly(ester amide)s: in vitro study using potentiometric titration, J Mater Sci.: Mater in Medicine 15: 185-190.
- N Zavradashvili, G Jokhadze, M Gverdtsiteli, G Otinashvili, N Kupatadze, et al. (2013) Amino Acid Based Epoxy-Poly(Ester Amide)s - a New Class of Functional Biodegradable Polymers: Synthesis and Chemical Transformations, J. Macromol. Sci., Part A, Pure & Appl. Chem 50: 449-465.
- Tem Kantaria, Ten Kantaria, S Kobauri, M Ksovreli, T Kachlishvili, et al. A new generation of biocompatible nanoparticles made of resorbable poly(ester amide)s, Ann. Agrarian Sci.(in press).
- Laurent S, Yahia LH (2013) Protein corona: applications and challenges, in: B. Martinac (Ed.), Protein-Nanoparticle Interactions, Springer-Verlag Berlin Heidelberg.
- T Memanishvili, N Zavradashvili, N Kupatadze, D Tugushi, M Gverdtsiteli, et al. (2014) Arginine-based biodegradable ether-ester polymers of low cytotoxicity as potential gene carriers, Biomacromolecules 15: 2839-2848.
- N Zavradashvili, C Sarisozen, G Titvinidze, Teng Kantaria, D Tugushi, et al. (2019) Library of Cationic Polymers Composed of Polyamines and Arginine as Gene Transfection Agents, ACS Omega 4: 2090-2101.
- D Kharadze, T Memanishvili, K Mamulashvili, T Omiadze, L Kirmelashvili1, et al. (2015) In Vitro Antimicrobial Activity Study of Some New Arginine-based Biodegradable Poly (Ester Urethane)s and Poly (Ester Urea)s, J. Chem. Chem. Eng. 9: 524.
- Tem Kantaria, Teng Kantaria, S Kobauri, M Ksovreli, T Kachlishvili, et al. (2016) Biodegradable nanoparticles made of amino acidbased ester polymers: preparation, characterization, and in vitro biocompatibility study. Appl. Sci. 6: 444.
- M Mudgil, N Gupta, M Nagpal, P Pawar (2012) Nanotechnology: a new approach for ocular drug delivery system, Int. J. Pharm. Pharm. Sci. 4: 105-112.
- A Díaz, LJ del Valle, D Tugushi, R Katsarava, J Puiggalí, et al. (2015) New poly(ester urea) derived from L-leucine: electrospun scaffolds loaded with antibacterial drugs and enzymes. Mater. Sci. Engin. C 46: 450-462.
- SK Murase, LP Lv, A Kaltbeitzel, K Landfester, LJ del Valle, et al. (2015) Amino acid-based poly(ester amide) nanofibers for tailored enzymatic degradation prepared by miniemulsion-electrospinning. RSC Adv. 5: 55006-55014.
- SK Murase, LJ del Valle, S Kobauri, R Katsarava, J Puiggalí, et al. (2015) Electrospun fibrous mats from a L-phenylalanine based poly(ester amide): Drug delivery and accelerated degradation by loading enzymes, Polym. Degrad. Stabil. 119: 275-287.
- LJ del Valle, L Franco, R Katsarava, J Puiggalí (2016) Electrospun biodegradable polymers loaded with bactericide agents. AIMS Molecular Science 3: 52.
- A Díaz, LJ del Valle, N Rodrigo, MT Casas, G Chumburidze, et al. (2018) Antimicrobial Activity of Poly(ester urea) Electrospun Fibers Loaded with Bacteriophages. Fibers 6: 33.
