An Unusual and Rare Cause of Pancreatitis
Areeb Khan1*, Hameed Ur Rehman1, Habib Ismail2
1 Nottingham University hospitals, England.
2 University hospital coventry, Warwickshire, England, UK.
*Corresponding Author: Areeb Khan, Core Medical Trainee 2, Nottingham University hospitals, England, Tel: 004407405230682 ; Fax: 004407405230682 ; E-mail:areebkhan11@hotmail.com
Citation: Areeb Khan, Hameed Ur Rehman, Habib Ismail (2022) An Unusual and Rare Cause of Pancreatitis. Gastroenterol Hepatol J 5: 123.
Copyright:© 2022 Areeb Khan, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received date: December 29, 2021; Accepted date: January 06, 2022; Published date: January 11, 2022.
Summary
An 82-year-old female presented to hospital with one-week history of transient loss of consciousness and intermittent back pain after starting furosemide. Four days after admission, her inflammatory markers were significantly raised. An MRI was obtained as discitis was suspected. This ruled out discitis but showed free fluid in the abdomen. A CT scan of her abdomen and pelvis was obtained which demonstrated evidence of acute pancreatitis. A serum amylase and lipase were obtained but these were normal. Given her acute presentation and prior literature attributing pancreatitis to furosemide, it was concluded that Furosemide was the cause of her pancreatitis. Her inpatient stay was uncomplicated and she was discharged with increased social support in the community.
Background
Pancreatitis is a common disease of the gastrointestinal tract. Its incidence is rising in the UK [1] and its acute form can be associated with a mortality rate as high as 30% [2,3]. As well as a timely diagnosis, management depends upon on establishing an underlying cause and treating it promptly and appropriately. Drug-induced pancreatitis (DIP) was previously noted to be extremely rare and almost a forgotten entity. Here, we describe a case of an elderly female who developed acute pancreatitis one week after initiating furosemide use.
Case Presentation
An 82-year-old female with a history of chronic kidney disease (CKD) stage II, chronic obstructive pulmonary disease (COPD) and stable angina was admitted to our acute medical unit with a one week history of multiple episodes of transient loss of consciousness (T-LOC) and intermittent back pain. One week prior to admission she had been initiated on furosemide 40 milligrams once a day for bilateral lower limb oedema.
Her medication history consisted of atorvastatin 20mg at night, seretide inhaler and aspirin 75mg once a day. She was a non-smoker, did not consume alcohol and lived alone at home without the support of carers.
On examination, she had a significant postural hypotension (sitting blood pressure ((BP)) – 137/55mmHg vs after 1 min of standing BP 66/36mmHg). Otherwise, there were no abnormalities noted on assessment of her cardiovascular, respiratory, abdominal, neurological and musculoskeletal systems
Her admission blood tests revealed a normocytic anaemia (Hb 11.4) and normal coagulation profile. Her inflammatory markers were slightly raised with a white cell count (WCC) of 10.1 and C-reactive protein (CRP) of 31. Her liver function tests revealed a slightly raised alkaline phosphatase (ALP) of 123 IU/l and she had a presumed acute kidney injury (AKI) with a urea of 10.6mmol/l and creatinine of 135 umol/l. No previous blood tests were available for comparison.
An initial diagnosis of postural hypotension secondary to furosemide was made. Her furosemide was stopped and she was reviewed by the occupational and physiotherapists.
Investigations
Four days after admission her inflammatory markers significantly increased (WCC 23.7 and CRP 181). As her back pain remained an issue and she reported no new symptoms, and a septic screen revealed no abnormalities, she was investigated for discitis. Whilst an MRI spine showed no evidence of discitis, it did demonstrate free fluid in the right flank and pelvis. A CT of her abdomen and pelvis was undertaken and this showed ill-defined, patchy low attenuation within the head and uncinated lobe of the pancreas with surrounding inflammatory change and evidence of focal haemorrhage. No evidence of gallstone disease was identified on CT or MRI. Figure 1.
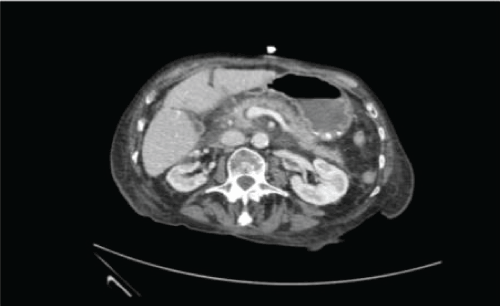
Figure 1: CT A-P demonstrating peri pancreatic fluid and fat stranding.
A serum amylase and lipase were obtained but these were normal. This patient’s Modified Glasgow Score was four, scoring on albumin of 24, a lactate dehydrogenase (LDH) of 983 U/L, her age and neutrophilia. An ultrasound of her abdomen was performed which did not demonstrate any gallstone disease or dilatation of her common bile, intra or extrahepatic bile ducts. This was confirmed with a magnetic resonance cholangiopancreatography (MRCP). She had normal calcium, parathyroid and triglyceride levels, a negative autoimmune and viral screen, no personal history of trauma or insect bites and no family history of pancreatitis.
Differential Diagnosis
After a multidisciplinary team meeting between the medical and surgical teams, it was concluded that in the absence of any other cause of acute pancreatitis being present, the recent introduction of furosemide was the probable cause of her Acute Pancreatitis (AP) – the likely reason for her admission to hospital.
Treatment
This patient was managed successfully with supportive measures including continuing cessation of the culprit medication, intravenous fluids and monitoring for complications. She was subsequently discharged from hospital a few days later with a package of care in the community and follow up with the surgical team.
Discussion
Introduction and Epidemiology of Pancreatitis Pancreatitis is defined as inflammation of the pancreas and can be classified as acute (AP) or chronic (CP) (if enough damage takes place and fibrosis, calcification and ductal inflammation ensues) [4]. We will focus on AP in this review though it is worth noting that patients with chronic pancreatitis can develop AP too. AP has an annual incidence of 150-420 cases per million population [3]. It is more common in the middle aged and the elderly but can occur at any age.
The pathophysiology of AP is not well understood but is related to the activation of digestive enzymes, such as protease, lipase and amylase, which results in cellular injury. This results in inflammation of the pancreas and its surrounding structures.
Some of the long term consequences of pancreatitis include necrosis and bacterial translocation from the gut [5].
Aetiology
Gallstones is the most common cause of AP accounting for 40% of it [6]. Gallstones is extremely prevalent with estimates of 10%-20% in the general population [7]. Alcohol is the second most common cause of AP in Europe. It is associated with drinking an excess of 80g of alcohol a day. Spirits have been strongly associated with alcoholic pancreatitis compared with beer and wine [2,8]. Smoking has been shown to be increase the risk of AP (risk of non-gallstone pancreatitis doubles with smoking) [9]. Other common or recognised causes include iatrogenic (secondary to Endoscopic retrograde pancreatography (ERCP)), hypertriglyceridaemia, hypercalcaemia, neoplastic disease, genetic predisposition, autoimmune pancreatitis, viruses such as Mumps, and drugs [10]. As demonstrated above, these causes were either excluded or very unlikely to be causative factors in our case.
Diagnostic criteria
The American Pancreatic Association (APA) states that two of the following criteria must be met for the diagnosis of AP [11]:
* Clinical
* Biochemical- amylase or lipase more than three times the upper limit of normal
* Radiographic- CT, MRI or Ultrasound criteria
Lipase and amylase are extremely sensitive in AP. Lipase has a negative predictive value between 94% and 100% whilst amylase has a negative predictive value of 99%. Amylase rises within the first 24 hours of onset and normalises within five days [12,13].
This could explain normoamylasaemia in this case as amylase was obtained seven days after admission (therefore, 14 days after onset of symptoms) [14].
This explanation is also applicable to the normal lipase level; although, lipase has a longer half-life. Lipase peaks within 8 hours and remains elevated for one to two weeks [12]. As lipase sample was obtained almost two weeks after onset of symptoms, the window could have been missed. This case also highlights the important role of imaging in the diagnosis of pancreatitis as the patient had an “incidental” finding of pancreatitis. The patient only met one APA criteria. The rationale behind normal lipase and amylase was explored. The absence of abdominal pain, biochemical markers of pancreatitis and features of gallstone disease or alcohol excess would have made it very challenging for pancreatitis to be a differential, particularly on admission to our hospital. It should be noted however that in a recent National Confidentiality Enquiry into Patient Outcome and Death (NCEPOD) report investigating the diagnosis and management of AP in the UK, back pain was noted to be part of the presenting complaint in 15% of cases [15]. A thorough history is all the more important, particularly in the elderly in whom atypical presentations are common.
Drug-Induced Pancreatitis
DIP is extremely rare and has an incidence of 0.1% to 2% [16]. However, the recent NCEPOD reported 17.5% cases of pancreatitis of unknown aetiology so whether the true incidence of DIP is as rare as we are led to believe remains in question [15]. The World Health Organisation (WHO) has listed 525 different drugs suspected to cause acute pancreatitis. Definite causality has been confirmed in 31 drugs. The reason behind such a reduced number demonstrating definite causality is that symptoms have to reoccur upon rechallenge [17]. Clinicians will deter against rechallenging the patient with a drug that is known to be harmful; especially in pancreatitis as it is associated with a high mortality [10]. Steroids, opiates, mercaptopurine, didanosine, asparginase and azathioprine are some of the common examples of drugs capable of causing acute pancreatitis [17]. Trivedi et al (2005) showed that there have been 21 reported cases of pancreatitis induced by furosemide, the suspected drug in this clinical scenario, and only three cases in which it was confirmed upon re-exposure [18]. The mechanism explaining DIP, including furosemide, is theoretical. Possible explanation of furosemide-induced pancreatitis includes direct toxic effect to the pancreas, induction of pancreatic enzymes and ischaemia. Ischaemia could be secondary to the decreased extracellular volume which reduces pancreatic blood flow [16]. Associating a drug with a reaction is difficult. The most established adverse drug reaction probability scale was described by Naranjo in 1981. A total score of 4 was calculated (presence of previous conclusive reports on this reaction, adverse event appeared after the suspected drug was administered and the improvement of the patient after the drug was discontinued) and this falls under the possible category.
Conclusion
DIP is extremely rare and has an incidence of 0.1% to 2% which makes it a forgotten entity [16]. Pancreatitis should be considered as a differential from furosemide use in patients with acute deterioration in physical health. There are four learning points about this unique case. Firstly, we report a rare case of furosemide-induced pancreatitis. Secondly, it is important to note that back pain, as in this case, is the presenting complaint in 15% of pancreatitis. Thirdly, normal amylase and lipase can occur in pancreatitis and the half –life of the tests should be taken into consideration during evaluation of the patient. Fourthly, imaging has a vital role in patients where the diagnosis is uncertain.
Learning Points
We report a rare case of furosemide-induced pancreatitis.
* It is important to note that back pain, as in this case, is the presenting complaint in 15% of pancreatitis.
* Normal amylase and lipase can occur in pancreatitis and the half –life of the tests should be taken into consideration during evaluation of the patient.
* Imaging has a vital role in patients where the diagnosis is uncertain.
References
- Yadav D, Lowenfels AB (2006) Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas 33: 323-330. [crossref]
- Lankisch PG, Apte M, Banks PA (2015) Acute pancreatitis. Lancet 386: 85-96. [crossref]
- Working Party of the British Society of Gastroenterology; Association of Surgeons of Great Britain and Ireland; Pancreatic Society of Great Britain and Ireland; Association of Upper GI Surgeons of Great Britain and Ireland (2005) UK guidelines for the management of acute pancreatitis. Gut 54 Suppl 3: iii1-9. [crossref]
- Klöppel G (2007) Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol 20: S113-131.
- Schmid SW, Uhl W, Friess H, Malfertheiner P, Büchler MW (1999) The role of infection in acute pancreatitis. Gut 45: 311-316. [crossref]
- Wang GJ, Gao CF, Wei D, Wang C, Ding SQ (2009) Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol 15: 1427-1430. [crossref]
- Stinton LM, Shaffer EA (2012) Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 6: 172-187. [crossref]
- Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA, et al. (2013) The incidence of acute pancreatitis: Impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther 38: 539-548.
- Sadr-Azodi O, Andrén-Sandberg Å, Orsini N, Wolk A (2012) Cigarette smoking, smoking cessation and acute pancreatitis: a prospective population-based study. Gut 61: 262-267. [crossref]
- Stevenson K, Carter CR (2016) Acute pancreatitis. Surg [Internet] 31: 292-300.
- Working Group IAP/APA Acute Pancreatitis Guidelines (2013) IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 13: e1-15.
- Cartier T, Sogni P, Perruche F, Meyniard O, Claessens YE, et al. (2006) Normal lipase serum level in acute pancreatitis: a case report. Emerg Med J 23: 701-702. [crossref]
- Lin XZ, Wang SS, Tsai YT, Lee SD, Shiesh SC, et al. (1989) Serum amylase, isoamylase, and lipase in the acute abdomen. Their diagnostic value for acute pancreatitis. J Clin Gastroenterol 11: 47-52.
- Clavien PA, Robert J, Meyer P, Borst F, Hauser H, et al. (1989) Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg 210: 614-620. [crossref]
- National Confidential Enquiry into Patient Outcome and Death. Treat the Cause - A review of the quality of care provided to patients treated for acute pancreatitis. 2016.
- Jones MR, Hall OM, Kaye AM, Kaye AD (2015) Drug-induced acute pancreatitis: a review. Ochsner J 15: 45-51. [crossref]
- Nitsche C, Maertin S, Scheiber J, Ritter CA, Lerch MM, et al. (2012) Drug-induced pancreatitis. Curr Gastroenterol Rep 14: 131-138. [crossref]
- Trivedi CD, Pitchumoni CS (2005) Drug-induced pancreatitis: an update. J Clin Gastroenterol 39: 709-716. [crossref]
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, et al. (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30: 239-245. [crossref]
