A Bond between Corona Virus and Diabetes Mellitus: Deadly cause for Public
Mihir Y. Parmar1,2*, Priya Panchal 3,4
1Head & Professor, Department of Pharmacology, Krishna School of Pharmacy & Research, KPGU, Vadodara, Gujarat, India.
2Principal, Director & Professor, Department of Pharmacology. Sanjay College of Pharmacy, Dr. A. P. J. Abdul Kalam Technical University, Mathura, UP, India.
3Assistant Professor, Department of Pharmaceutics, Sanjay College of Pharmacy, Dr. A. P. J. Abdul Kalam Technical University, Mathura, UP, India.
4Research Scholar, Department of Pharmaceutics, NIMS University, Jaipur, Rajasthan, India.
*Corresponding Author: Mihir Y. Parmar, Head & Professor, Department of Pharmacology, Krishna School of Pharmacy & Research, KPGU, Vadodara, Gujarat, India, Principal, Director & Professor, Department of Pharmacology. Sanjay College of Pharmacy, Dr. A. P. J. Abdul Kalam Technical University, Mathura, UP, India., Tel: +91-7016422726; Fax: +91-9638330050; E-mail: mihirparmar4uonly@yahoo.com
Citation: Mihir Y. Parmar and Priya Panchal (2023) A Bond between Corona Virus and Diabetes Mellitus: Deadly cause for Public. Arch Mol Med & Gen 3: 120.
Received: April 6, 2023; Accepted: April 17, 2023; Published: April 20, 2023.
Copyright: © 2023 Mihir Y. Parmar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The deadly disease of Corona Virus disease (COVID-19), a disease caused by severe acute respiratory syndrome Corona Virus 2 (SARSCoV-2), is causing extensive morbidity and mortality. Elder person and presence of diabetes mellitus, hypertension, and obesity significantly increases the warning for hospitalization and death in COVID-19 patients. In this point of view, clued-up by the studies on SARS-CoV-2, Middle East respiratory syndrome (MERS-CoV), and the existing literature on SARS-CoV-2, we will discuss probable mechanisms by which diabetes modulates the host-viral interactions and host-immune responses. We look forward to bringing to light gaps in knowledge that require further studies applicable to COVID-19 in patients with diabetes.
Keywords
Bond, Corona Virus, Diabetes Mellitus, Epidemic, Obesity.
Introduction
Corona viruses (CoV) are enveloped viruses with a single stranded, positive-sense RNA genome recognized to cause respiratory infections in humans [1, 2]. In broad-spectrum, in most immune competent individuals, human CoV infection led to mild upper respiratory infection. However, two highly pathogenic CoV have resulted in outbreaks of severe acute respiratory syndrome (SARS) in 2003 in Guangdong, China and Middle East respiratory syndrome (MERS) in Middle Eastern countries a decade later. SARS-CoV and MERS-CoV were identified to cause SARS and MERS, respectively. In December 2019, a novel corona virus, SARS-CoV-2, was identified as the pathogen causing corona virus disease (COVID-19) in Wuhan, China [3-5]. On March 11, 2020, COVID-19 was declared a pandemic by the WHO. As of March 27, 2020, there have been a total of 103,942 confirmed cases with 1689 deaths in the US Globally, 27,324 deaths have been reported among 595,800 confirmed cases [6].
Individuals with diabetes mellitus (DM), hypertension, and severe obesity (BMI 40 kg/m2) are more likely to be infected and are at a higher risk for complications and death from COVID-19 [7, 7a 2a]. Interestingly, there was a similar increased risk for SARS and MERS in individuals with DM. In the US, 34.2 million or 10.5% of the total population have DM. Among those aged 65 years or older, a population at higher risk for death from COVID-19, 26.8% has DM [8]. Hypertension and severe obesity are present in 68.5% and 15.6% of individuals diagnosed with DM, respectively. Over a period of months, a substantial portion of the US population will be infected by SARS-CoV-2. Although a significant number will remain asymptomatic and be able to transmit the virus, the estimated proportion of symptomatic individuals requiring hospitalization increases with age.
In individuals older than 60 years, that proportion ranges from 20-30%. Furthermore, in this older group, the percentage of hospitalized patients requiring care in an intensive care unit (ICU) is 27–71% with an infection fatality rate (IFR) ranging from 2.2-9.3% [9]. Although these estimates are preliminary and likely to change, considering the prevalence of DM, hypertension, and severe obesity in the US and the substantial increased risk for COVID-19 and its complications in patients with these conditions, it is likely the pandemic has the potential to cause significant mortality and morbidity. Specialists and health care providers will be providing clinical care to many patients with COVID-19 in inpatient, outpatient, and tele health settings. Increased awareness of the clinical features, pathophysiology, and potential mechanisms that increase the risk is needed to provide better care and prompt new investigations, both basic and clinical, to better understand COVID-19 in patients with diabetes.
Clinical features and Natural Course of COVID 19: The median age of patients infected with SARS-CoV-2 is in the range of 48–58 years, men comprise more than half of the cases, the average incubation period is 5 days, and 98% of those who develop symptoms will do so within 11.5 days [7]. The clinical manifestations of COVID-19 vary and include the asymptomatic carrier status, acute respiratory disease (ARD), and pneumonia [7]. The prevalence of asymptomatic cases is significant (20-86% of all infections) and are defined as individuals with positive viral nucleic acid tests, but without any COVID-19 symptoms [10-1. Transmission rates and respiratory viral load in asymptomatic carriers are similar to symptomatic patients [11], partially explaining the rapid spread of SARS-CoV-2. In addition to a laboratory-confirmed COVID-19 diagnosis, patients with ARD manifest with fever, fatigue, respiratory (cough, dyspnea) or gastrointestinal (nausea, diarrhea, vomiting) symptoms, and no significant abnormalities on chest imaging [7, 12]. Patients with pneumonia have respiratory symptoms and positive findings in chest imaging. Severe pneumonia can present as acute respiratory distress syndrome (ARDS), leading to severe hypoxia, respiratory failure, multi organ failure, shock, and death [7, 12].
Pathophysiology of SARS-CoV-2 infection: The genetic sequence of SARS-CoV-2 showed more than 80% shared identity to SARS-CoV and 50% to the MERS-CoV, and both SARS-CoV and MERS-CoV originate in bats and infect humans and wild animals [1, 13]. Cellular CoV entry is a complex process that involves receptor binding and proteolysis leading to virus-cell fusion. CoV is made up of four structural proteins: spike (S), membrane (M), nucleocapsid (N), and envelope (E) proteins. The S protein mediates receptor binding on the host cell membrane through the receptor-binding domain (RBD) in the S1 domain and membrane fusion through the S2 subunit [14-15]. Angiotensin-converting enzyme 2 (ACE2) is the cellular receptor for SARS-CoV and SARSCoV-2, in contrast to MERS-CoV, which utilizes dipeptidyl peptidase 4 (DPP4) as its cellular receptor [16-17] (Fig. 1).
Figure 1: Cellular entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
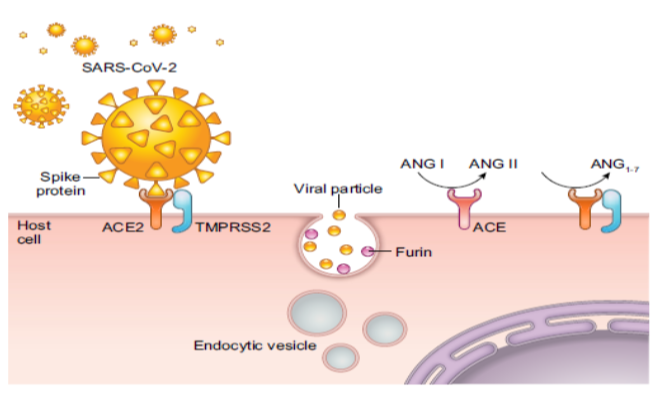
(Description of Figure 1: The initial step in cellular entry of the virus is the binding of SARS-CoV-2 spike protein to cell surface angiotensin converting enzyme 2 (ACE2). Cellular proteases such as TMPRSS2 and furin are involved in priming of the S protein, which involves cleavage at the S1/S2 domains. This allows the fusion of the virus to the cell surface. Virions are taken up into endosomes, where SARS-CoV-2-S is cleaved and possibly activated by the pH-dependent cysteine protease cathepsin L. Once inside the cell, SARS-CoV-2 uses the endogenous cellular machinery to replicate itself. ACE catalyzes the conversion of angiotensin Ang I to the octapeptide Ang II, whereas ACE2 converts Ang II to Ang 1–7. Ang II through the activation of Ang II type 1a receptors induces vasoconstriction and proliferation, whereas Ang 1–7 stimulates vasodilatation and suppresses cell growth.)
This interaction thus determines host tropism and ultimately clearance of the virus. ACE2 is expressed in the upper respiratory system, type I and II alveolar epithelial cells in the lungs, the heart, endothelial cells, kidney tubular epithelium, enterocytes, and the pancreas [16]. After binding to ACE2, proximal serine proteases such as TMPRSS2 are involved in S protein priming and cleavage of the spike (Fig. 1). Proteases such as Furin subsequently release the spike fusion peptide, and the cellular virus enters through an endosomal pathway [14-15]. The low pH and presence of proteases such as cathepsin-L characteristic of the endosomal microenvironment favor the delivery of SARS-CoV-2 genome into the cytosol where further viral replication leads to the formation of mature virions and subsequent spread.
Infected cells undergo apoptosis or necrosis and trigger inflammatory responses marked by the activation of proinflammatory cytokines or chemokines, which leads to the recruitment of inflammatory cells. CD4 T helper (Th1) cells regulate antigen presentation and immunity against intracellular pathogens such as CoV through interferon gamma (IFN-?) production. Th17 cells induce the recruitment of neutrophils and macrophages by producing interleukin-17 (IL-17), IL-21, and IL-22 [18]. SARS-CoV-2 infects circulating immune cells and increases apoptosis of lymphocytes (CD3, CD4, and CD8 T cells), leading to lymphocytopenia. Indeed, the degree of lymphocytopenia is associated with the severity of SARS CoV-2 infection [7, 19, 20a].
Lower T cell function relieves the inhibition on innate immune system leading to secretion of high amounts of inflammatory cytokines in what is known as a “cytokine storm” [7a]. In fact, circulating levels of cytokines/chemokines such as Interleukin (IL-6), tumor necrosis factor-α (TNF-α), chemokines CXC-chemokine ligand 10 (CXCL10) and CC-chemokine ligand 2 (CCL2) involved in the cytokine storm syndrome are elevated and may play a role in SARSCoV-2-driven hyperinflammation leading to multiorgan failure [20-22].
Potential Mechanisms that increase the risk of COVID-19 in Diabetes: It is now well documented that older age and the presence of DM, hypertension, and severe obesity (BMI 40 kg/m2) increase morbidity and mortality in patients with COVID-19 [7-8]. Considering the high prevalence of cardiovascular disease (CVD), obesity, and hypertension in patients with DM, it is unknown whether DM independently contributes to this increased risk. However, plasma glucose levels and DM are independent predictors for mortality and morbidity in patients with SARS [23]. Potential mechanisms that may increase the susceptibility for COVID-19 in patients with DM include: 1) higher affinity cellular binding and efficient virus entry, 2) decreased viral clearance, 3) diminished T cell function, 4) increased susceptibility to hyperinflammation and cytokine storm syndrome, and 5) presence of CVD (Fig. 2).
Figure 2: Putative Mechanisms contributing to increased susceptibility for Coronavirus disease (COVID-19) in patients with Diabetes Mellitus (DM).
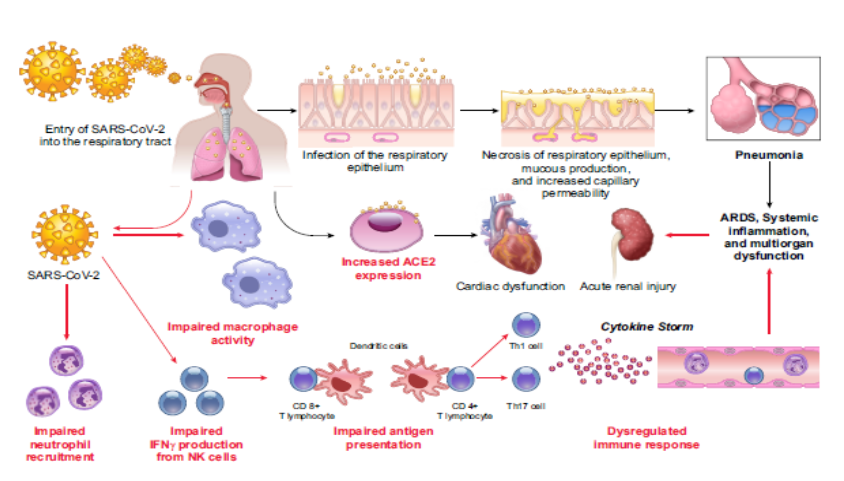
(Description of Figure 2: Following aerosolized uptake of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), invasion of the respiratory epithelium and other target cells by SARS-CoV-2 involves binding to cell surface angiotensin converting enzyme 2 (ACE2). Increased expression of ACE2 may favor more efficient cell binding and entry into cells. Early recruitment and function of neutrophils and macrophages are impaired in DM. Delay in the initiation of adaptive immunity and dysregulation of the cytokine response in DM may lead to the initiation of cytokine storm). Augmented ACE2 expression in alveolar AT2 cells, myocardium, kidney, and pancreas may favor increased cellular binding of SARS-CoV-2 [24-25]. Increased expression of ACE2 has been demonstrated in the lung, kidney, heart, and pancreas in rodent models of DM. Insulin administration attenuates ACE2 expression [26-27], while hypoglycemic agents such as glucagon-like peptide-1 (GLP-1) agonists (liraglutide) and thiazolidinediones (TZDs; pioglitazone, antihypertensives such as ACE inhibitors, and statins upregulate ACE2 [28-29]. Until recently, whether DM was causally linked to ACE2 expression levels in the lung in humans was unknown. Using a phenome-wide Mendelian randomization study, Rao et al. [30] explored diseases or traits that may be causally linked to increased ACE2 expression in the lung. Interestingly, they found that DM was causally associated with increased lung ACE2 expression. Circulating levels of furin, a cellular protease involved in facilitating viral entry by cleaving the S1 and S2 domain of the spike protein, are elevated in patients with DM [31]. These studies support the hypothesis that patients with DM are susceptible to SARS-CoV-2 infection. Indeed, a recent study reported that clearance of SARS-CoV-2 was delayed in patients with DM, a finding that needs to be confirmed in larger studies (Fig. 2). ACE catalyzes the conversion of the prohormone, angiotensin (Ang I to the octapeptide, AngII), whereas ACE2 converts Ang II to Ang 1–7. Ang II, through the activation of Ang II type 1a receptors induces vasoconstriction and proliferation, whereas Ang 1–7 stimulates vasodilatation and suppresses cell growth (Fig. 1). Increased ratio of pulmonary ACE/ACE2 activity as observed in patients with ARDS [32] favors AngII generation. Once bound to ACE2, SARSCoV downregulates cellular expression of ACE2, and the unopposed action of Ang II contributes to acute lung injury [33]. Binding to ACE2 alone does not lead to severe lung injury as is observed with other CoVs (NL63) [13, 34]. Whether SARS-CoV-2 causes down regulation of pulmonary ACE2 is unknown. Nevertheless, there exists a potential for salutary, if not therapeutic, effects of Ang II receptor blockers, ACE inhibitors, TZDs, GLP-1 agonists, and statins in the setting of low ACE2 expression. Lacking further evidence of risk or benefit, the American College of Cardiology, the American Heart Association, and the American Society of Hypertension have recommended that patients should continue treatment with their usual antihypertensive therapy [35]. DM inhibits neutrophil chemotaxis, phagocytosis, and intracellular killing of microbes. Impairments in adaptive immunity characterized by an initial delay in the activation of Th1 cell-mediated immunity and a late hyperinflammatory response are often observed in patients with diabetes [36, 2a]. In an elegant study, Kulcsar et al and Mihir Y Parmaret al [37, 38] examined the effects of DM in a humanized mouse model of MERS-CoV infection on a high-fat diet. Following MERS-CoV infection, the disease was more severe and prolonged in diabetic male mice and was characterized by alterations in CD4 T cell counts and abnormal cytokine responses (elevated IL17a). Consistent with this finding, in patients with COVID-19, peripheral counts of CD4 and CD8 T cells are low, but with a higher proportion of highly proinflammatory Th17 CD4 T cells, as well as elevated cytokine levels. Thus, it is likely that patients with DM may have blunted anti-viral IFN responses, and the delayed activation of Th1/Th17 may contribute to accentuated inflammatory responses (Fig. 2).
Conclusion
There is a scarceness of data in the US regarding co morbidities and COVID-19 outcomes and mechanisms that modulate viral pathogenesis. Certain racial groups such as African Americans, Hispanics, Asians, and Native Americans are highly prone to develop DM, and disparities in health care make these groups more vulnerable. Identification of clinical and biochemical parameters using multiomics approaches that predict severity of the COVID-19 in DM using large data sets is urgently needed. Studies in humanized ACE2 (hACE2) mice and non-human primates aimed at understanding how hyperglycemia, hyperinsulinemia, and hypoglycemic agents affect pathogenesis of COVID-19 and how DM affects the efficacy of vaccines and antiviral investigational agents currently in trials are warranted. Finally, we need to develop novel ways to deliver care to our patients with DM using tele health, remote patient monitoring, and wearable technologies. As the global pandemic unfolds and rapidly spreads across the United States, social isolation measures will enable the transition, but there is an urgent need for basic and clinical investigations to address the many important and unanswered questions.
Acknowledgments
The authors acknowledge and heartly thanks the health care professionals taking care of patients with COVID-19 from their heart.
Grants: This work was supported in part by the Lion’s Group of Diabetes, USA
Disclosures: No conflicts of interest, financial or otherwise, are declared by the authors.
Author Contributions
Version of manuscript. MYP and PP collected data and done review of literature. MYP and PP prepared figures and drafted manuscript; MYP and PP edited and revised manuscript; MYP approved final.
References
- Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17: 181-192.
- u S et al. (2016) Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol 24: 490-502.
- Mhir YP (2020) Pandemic of Novel Coronavirus (n Covid 19): Impact on World. International Journal of Clinical Studies & Medical Case Reports 1: 1-6.
- Drosten C et al. (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348: 1967–1976.
- Zaki AM et al. (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367: 1814 –1820.
- Zhong NS et al. (2003) Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February 2003. Lancet 362: 1353–1358.
- Johns Hopkins Coronavirus Resource Center. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE).
- Guan WJ et al. (2019) China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease in China. N Engl J Med (In press).
- 7a. Palm NW, Medzhitov R (2007) Not so fast: adaptive suppression of innate immunity. Nat Med 13: 1142–1144.
- 7b. Parmar MY et al. (2020) Superpower of antioxidant in oxidative stress and diabetes mellitus. Diabetes Updates: 6 1-4.
- Centers for Disease Control and Prevention (2020) National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services.
- Ferguson N et al. Imperial College of London COVID-19 Response Team. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand.
- Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. In press.
- Li R et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science (In press).
- Wang D et al. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061.
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med.
- Hoffmann M et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell.
- Walls AC et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell.
- Li W et al. (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450 –454.
- Raj VS et al. (2013) Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus- EMC. Nature 495: 251–254.
- De Wit E et al. (2016) SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 14: 523–534.
- Wu C et al. (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med.
- Gao Y et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 25770.
- 20a. Mihir YP, Tribhuvan S and Akhlesh V (2020) Pandemic of Novel Coronavirus (COVID-19): Impact on World. International Journal of Clinical studies and Medical Case reports: 2: 1-6.
- Mehta P et al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395: 1033– 1034.
- Wan S, Yi Q et al. (2020) Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). MedRxiv.
- Yang JK et al. (2006) Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med 23: 623–628.
- Liu F et al. (2020) Highly ACE2 expression in pancreas may cause pancreas damage after SARS-CoV-2 infection.
- Zou X et al. (2019) Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med (In press).
- Roca-Ho H et al. (2017) Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse. Int J Mol Sci 18: 563.
- Wysocki J et al. (2006) ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139.
- Ferrario CM et al. (2005) Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin- converting enzyme 2. Circulation 111: 2605–2610.
- Romaní-Pérez M et al. (2015) Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of Type 1 diabetes rats. Endocrinology 156: 3559–3569.
- Rao S and Lau A (2020) Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of 2019- nCov: A Mendelian randomization analysis.
- Fernandez C et al. (2018) Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med 284: 377–387.
- Wösten-van Asperen RM et al. (2013) Imbalance between pulmonary angiotensinconverting enzyme and angiotensin-converting enzyme 2 activity in acute respiratory distress syndrome. Pediatr Crit Care Med 14: 438-441.
- Kuba K, Imai Y et al. (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879.
- Su S et al. (2016) Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol 24: 490–502.
- Danser AHJ, et al. (2020) Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension 120: 12015082.
- Kulcsar KA et al. (2019) Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 4: 4.
- Hodgson K et al. (2015) Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 144: 171–185.
- Mihir Y Parmar (2020) Impact of Novel Coronavirus (nCOVID-19) on Mental Health: Impairment of Brain Activity. BioMed ResearchJournal 4: 260-262.
