Sero-Prevalence Investigation of Peste Des Petits Ruminants (PPR) and Associated Risk Factors in Libya During 2015-2016
Abdusalam Sharef Mahmoud1,2,3*, Daria Di Sabatino1, Maria Luisa Danzetta1, Francesco Tolari3, Mario Forzan3, Maurizio Mazzei3, Abdunaser Dayhum2, Federica Monaco1
1L'Istituto ZooprofilatticoSperimentale dell'Abruzzo e del Molise "Giuseppe Caporale" (IZSAM), Teramo, Italy.
2Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya.
3Department of Veterinary Science, University of Pisa, Pisa, Italy.
*Corresponding Author: Abdusalam Sharef Mahmoud,L'Istituto ZooprofilatticoSperimentale dell'Abruzzo e del Molise "Giuseppe Caporale" (IZSAM), Teramo, Italy, Tel: +218 91 6328135; Fax: +218 21 4628421; E-mail: alawiym@yahoo.com
Citation: Abdusalam Sharef Mahmoud, Daria Di Sabatino, Maria Luisa Danzetta, Francesco Tolari, Mario Forzan, et al. (2020) Sero-Prevalence Investigation of Peste Des Petits Ruminants (PPR) and Associated Risk Factors in Libya During 2015-2016. Medcina Intern 4: 143.
Copyright: © 2020 Abdusalam Sharef Mahmoud, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 09, 2020; Accepted: January 16, 2020; Published: January 21, 2020.
Abstract
Peste des Petits Ruminants (PPR) is considered one of the most important transboundary animal diseases (TADs) with socio-economic impacts on national and international levels. During 2015-2016 a total of 690 serum samples were collected from unvaccinated domestic ruminants of which 555 sheep, and 135 goats representing teen provinces distributed in four Libyan branches (Green Mountain, Benghazi, West Mountain and Sabha). The sample were analysed at IZSAM, Teramo, Italy, by using competitive ELISA, IDvet innovative diagnostics (IDvet, 310 rue Louis Pasteur-34790 Grabels, France). The overall sero-prevalence rate (SPR) of PPR antibodies was estimated to be 41% (95% CL: 36% to 46%) among sheep and 39% (95% CL: 28% to 45%) among goat. A chi-square test was used to evaluate the probability of differences observed among SPR of infection. The results showed that the SPR of PPR was significantly (P= 0.015) higher in adult animals 41% (95% CL= 37%-46%) than in the young 24% (95% CL= 17%-32%). The highest SPR 75% (95% CL= 61%-85%) was recorded in Sabha province (Southern Libya) which highlighted statistically difference (P= 0.00001). The preliminary results of the present study could be useful to better focus on specific area of Libya to improve understand and evaluate the risk factors for disease spreading and to plan disease control activities as requested by FAO/OIE (PPR Global Control and Eradication Strategy).
Keywords
Keywords: PPR, Competitive ELISA, Sero-Prevalence, Risk Factors
Introduction
Peste des Petits Ruminants (PPR), also known as goat plague, is an acute contagious viral disease affecting sheep and goats and occasionally wild ruminants. The virus (PPRV) is classified in the genus Morbillivirus, family Paramyxoviridae [1]. The genome is represented by a non-segmented, negative, single-stranded RNA virus that encodes six structural proteins; nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin protein (H), RNA-dependent RNA polymerase (L), and two non-structural proteins (V and C).PPRV shares structural and biological characteristics with other members of the genus Morbillivirus, including, measles virus (MV), canine distemper virus (CDV), rinderpest (RP) virus, phocine-distemper virus (PDV),dolphin and porpoise morbilliviruses [1,3]. The disease was first identified in early 1940s in Côte d’Ivoire, West Africa [2]. All PPRV strains belong to a single serotype, but the different strains have been grouped into four distinct lineages, based on the sequence of a short (approximately 300 bases) fragment of the F, N and H genes [4-7]. Lineages I and II occur in West Africa, lineage III in East Africa in the Middle East and in southern India, and lineage IV in Asia [8] in Sudan [6,9], in Egypt [10], in Morocco [11], in Eritrea [12],in Algeria [13], in Tunisia [14] and more recently in Ethiopia [15]. Nowadays PPRV or specific antibodies have been detected in almost all African countries except for vast territories across Southern Africa [7].
The epidemiological situation of PPR in Libya is not clear. According to the results produced in the FAO project TCP/RAB/3302, the mean serological prevalence rate in 2013 was 34.5%, but to our knowledge during the subsequent years no official reports of the disease have been submitted to OIE.
The occurrence of PPR on the border of Europe could be considered a potential risk of introduction since the disease has not been introduced yet. The potential impact of introduction of the PPRV into European Union is supported by an experimental study in which a PPR Kurdish strain which belonging to Asian lineage IV inject into European goats was highly virulent and able to spread easily to in-contact animals [16].
PPR is considered one of the most important TADs with socio-economic impacts on the national and international levels and it is listed in the OIE Terrestrial Animal Health Code. According to OIE and FAO reports, each year, the disease causes economic losses estimated in 1.2 to 1.7 billion USD, due to animal deaths, reduced production and costs to control and to prevent the disease. Approximately a third of the financial impact occurs in Africa and a quarter in South Asia. Due to the increase of geographic expansion of PPR and the potential threat of PPR for food safety, FAO in collaboration with OIE, have launched a PPR Global Strategy (PPR-GS) to control and eradicate the disease. Specific objectives of the PPR-GS are the eradication of PPR by 2030. Therefore, objectives of our study were to investigate the SPR of PPR and associated risk factors for disease spreading and to plan disease control activities as requested by FAO/OIE (PPR Global Control and Eradication Strategy).
Material and methods
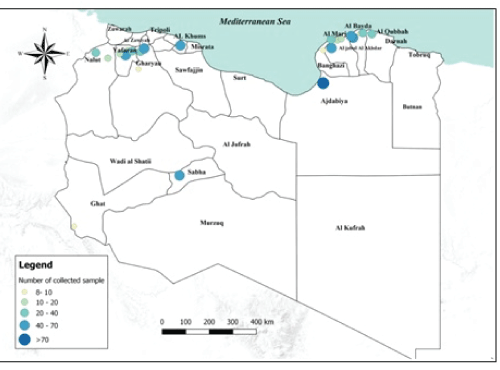
Sample collection: During 2015-2016 a total of 690 serum samples were collected from small ruminants of which 555 sheep, and 135 goats. In each farm a maximum of 10 serum samples were gathered regardless the herd size. Information on sex and age (expressed in months) were recorded. The samples were collected from the 96 farms with average herd size of, 255 sheep and 54 goats respectively. The farms were geographically distributed in teen provinces of Libya, in the following branches: Green Mountain, Benghazi, West Mountain and Sabha (Figure 1). Due to the national socio-political situation, the samples were collected only where the sampling activity was feasible and safe. To the best of our knowledge, all the samples in the study were collected from not PPR vaccinated animals.
Sample Analyses: Sheep and goats’ sera samples were tested by Competitive Enzyme-Linked Immunosorbent Assay (c-ELISA) using the commercial kit ID screen® PPR Competition (IDvet, 310 rue Louis Pasteur-34790 Grabels, France). The kit is validated for the detection of antibodies against PPRV in sheep and goats serum and plasma [17]. The test used was developed by a FAO reference laboratory (CIRAD-EMVT, Montpellier, France).
Statistical Analyses: The ages of animals were grouped in two classes (between 7 and 23 months), and adults (more than 24 months). Statistical analysis was performed using XLSTAT. For each proportion the prevalence and 95% confidence intervals (CL) were calculated using the Bayesian approach of Beta distribution. Association between the outcome variables (status of PPR infection in small ruminants) and its potential risk factors were screened in a univariable analysis using chi-square test. A p-value <0.05 was considered to be significant. A multivariate logistic regression has been performed using R software.
Figure 1: Number of collected samples represented by sampled areas and by sample size classes in Libya.
Results
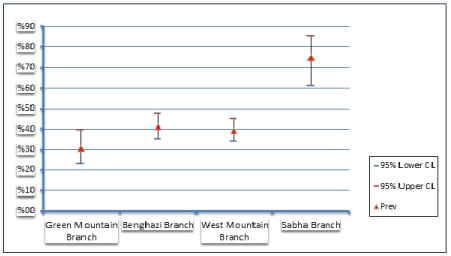
282 out of 690 small ruminant tested positive for PPR using c-ELISA (SPR=41%; CL 95%= 37%-45%). The SPR of PPR antibodies was estimated to be 42% (CL 95%= 36%-46%) among sheep and 39% (CL95%= 28%-39%) among goats. In the sampled Libyan branches, the SPR was not uniformly (P=0.00001) distributed (Figure 2). The highest and lowest SPR were reported in Sabha branch 75% (95% CL= 61%-85%), and Green mountain branch 31% (95% CL= 20%-40%) respectively (Figure 3).
Figure 2: The overall sero-prevalence and spatial distribution of Peste des Petits Ruminants according to Libyan branches.
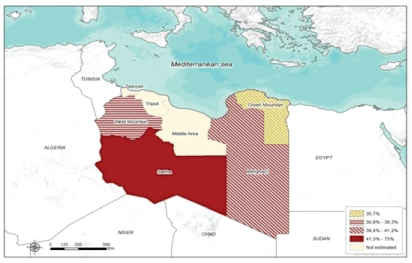
Figure 3: Seroprevalence of Peste des Petits Ruminants according to Libyan branches.
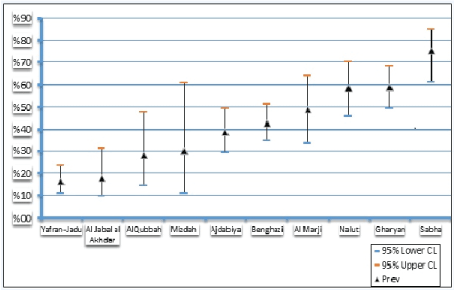
Also according to the Libyan provinces, the highest and lowest SPR of PPR were recorded in Sabha 75% 95% CL= 55%-85%) province and Yafran-Jadu province 16% (95% CL= 9%-23%) respectively (Figure 4). The reliability of results is supported by the high diagnostic specificity (99.4%) and sensitivity (94.5%) of the competitive ELISA used for detection of PPRV antibodies [17].
Figure 4: Sero-prevalenceof Peste des Petits Ruminants according to Libyan provinces.
The results of univariate analysis showed that, the independent variables including, sex, age class, farmed species were statistically significant, the SPR of PPR among different age groups of sheep and goats was (P= 0.015) significantly higher in adults animals 41% (95% CL= 37%-46%) than in the young ones 24% (95% CL= 17%-32%). Farmed species according to our findings is positive correlated with PPR prevalence, the highest value was recorded among mixed flock (43%). This is in agreement with several authors who had reported that goats are more susceptible to PPR than sheep [18] in Northern Jordan. Likewise [19], therefore the presence of goats in a sheep farm can increase the risk of PPR transmission. Even if sex, age and farmed species resulted all separately positive correlated with PPR infectious status in the univariate analysis, the multivariate analysis confirmed that only the age class was an independent risk factors for PPR.
Discussion
PPR is one of the priority diseases of the FAO-OIE Global Framework for the Progressive Control of Transboundary Animal Diseases (GF-TADs) with the purpose of eradicating the disease by 2030.
The epidemiological patterns of PPR in Africa have been changed specially in North African countries, when disease emerged for the first time in Morocco in 2008 with high case fatality rate [20]. In the following years, incursions of PPRV were reported almost in all Maghreb Region (MR). In 2011, both Tunisia and Algeria reported epizootic of PPR [14,21]. From 2012 to 2014 outbreaks were confirmed in Tunisia, Algeria and Mauritania [5]. Therefore, by ends of 2014 all countries of MR reported PPR outbreaks in their territories.
In Libya, according to the results produced in the FAO project TCP/RAB/3302 conducted in 2013, the overall SPR among small ruminants was 33% (95% CL= 31.4%-34.5%), the mean SPR was 34.5% (25% in local animals and 45% in imported heads from e.g. Sudan, Chad, Mali; 25% in animals < 12 months and 41% in animals older than two years). In the present study, the overall SPR of PPR in small ruminants was 41% (95% CL= 37%-45%), agreeing with FAO result [22]. Our results showed that, the PPR SPR among sheep and goats is higher than those reported by [14,23] in Tunisia (sheep 5.7% and goats 11.8%), [13] in Algeria (34.37% in goats; 20% in sheep), [20] in Morocco, [24] in Mauritania, and [25-27] in Turkey.
In 2013 and 2014 PPR circulation was reported in Tunisia with a mean SPR of infected herds of 46%, similar to the one recorded in the present study (SPR=41%; 95% CL=37%-45%). More recently PPR outbreaks were reported in Tunisia, with high case fatality rate 66% among of sheep farms [13]. No clinical signs were reported in the sampled animals of the present study. Nowadays no data are available regarding the PPR distribution and clinical outbreaks at national level as no national surveillance plan is in place. The highest value of PPR sero-prevalence (43%) was recorded among mixed flock. This agrees with several authors who had reported that goats are more susceptible to PPR than sheep [18,28] in northern Jordan, as well as similarly to the study reported by Kardjadj et al. [13], therefore, the presence of goats in a sheep farm can increase the risk of PPR transmission. The sex, age and farmed species resulted all separately positive correlated with PPR infectious status in the univariate analysis.
In the sampled Libyan branches with significantly (P= 0.00001), the highest SPR was observed in Sabha branch 75% (95% CL=61%-85%) as compare to all sampled branches (38%), and is considering higher than (56.8%) of PPR sero-prevalence that was reported during 2013-2014 [22]. The sero-prevalence results of PPR among different age groups of sheep and goats was showed, a SPR of 24% and 41% in small ruminants (SR) between 1 and 2 years and more than 2 years, respectively, this is a common result in serological studies as older animal have more chance to get in contact with infectious agent than younger animals [13,18,29-31].
The results also confirmed that, the southern region of Libya is a high-risk area and could be consider the main entrance for many TADs. However, the epidemiology of PPRV in Libya is not well understood, and, most importantly there were no data available regarding the molecular characterization of PPRV lineages circulating in Libya. Moreover, no official reports have been submitted to OIE from 2013 up to now. Therefore, to more understanding and updating epidemiological situation of PPR in Libya, in our study efforts have been made to provide preliminary data of these SPR and risk factors associated with PPR in sheep and goats in the different provinces of Libya. Nevertheless, highest SPR was observed in Sabha province, (Sabha branch) southern region, despite the small sample size, yet, it is important to underline that the samples were collected only in province of Sabha from seven farms. Therefore, our results could be biased by systematic errors in the sampling procedure and could not be representative of the entire southern region. This hypothesis is supported considering the legal and illegal movements of small ruminants into Libya which are concentrated in the southern provinces, that characterized by highly livestock commercial activities.
Indeed, the SPR recorded in Sabha province could be explained by the increasing chance of contact with imported infected animals in the southern region (Sabha branch). However, it is well know that, the difference among SPR to PPR from one region to another may be attributed to some factors including patterns of disease occurrences, livestock management, sheep and goats husbandry practices, the biosecurity measures, and levels of immunity [5,29]. According to OIE report in 2011, variation in SPR is probably related to the intensity of movement of livestock and trade of small ruminants. In addition, the higher sero-prevalence variation exits between the regions could be due to the sample size or may be attributed to the number of PPR affected animals from which blood samples were collected, as well as geographical differences. Even though, the sampled animals could not represents all the entire regions of the country to draw an epidemiological picture of the disease in the country, but, if we bring all our results together, it’s clearly that, from the epidemiological point of view, the present study results confirmed the constant of the endemicity of PPR in Libya. Therefore, further studies are needed to better understand the role of these risk factors in disease occurrence.
Conclusion
In all MR (Algeria, Morocco, Tunisia, Libya and Mauritania) PPR outbreaks might be under-detected and misdiagnosed as veterinary services not always compliance with OIE standards and high quality diagnostic testing and technical knowledge have been recently strengthened to perform PPR diagnosis. Considering how the spatial distribution and epidemiological patterns of PPR dramatically changed in MR in the recent years, all countries of MR must join forces to control and prevent PPR spread. The preliminary results of the present study could be useful to better focus on specific area of Libya to better understand the risk factors for disease spreading and to plan disease control activities as requested by FAO/OIE (PPR Global Control and Eradication Strategy).
Conflict of interest
All authors state that there is no conflict of interest.
Acknowledgment
The Authors would like to express their appreciations to staff of the Libyan National Center for Animal Health (NCAH) for their collaboration, facilitating and samples sending in time, especially to those who contributed in collecting samples and filling questionnaires. The authors would like to express their gratitude to the staff of the National Reference of Istituto Zooprofilattico Sperimentale dell' Abruzzo e del Molise G Caporale (IZSAM), Teramo, Italy, for providing fund and samples testing.
References
- Gibbs EP, Taylor WP, Lawman MJ, Bryant J (1979) Classification of peste des petitsruminants virus as the fourth member of the genus Morbillivirus. Intervirology11: 268-274.
- Gadennec L, Lalanne (1942) La peste des petits ruminants. Bulletin des Services Zoo Technique et des Epizootie de l’Afrique-Occidentale Franc¸aise 5: 16-21.
- Barrett T, Visser I, Mamaev L, Goatley L, Bressem MF, et al. Van Osterhaus A. Dolphine and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology 193: 1010-1012.
- Kumar KS, Babu A, Sundarapandian G, Roy P, Thangavelu A, et al. (2014) Molecular characterisation of lineage IV peste des petitsruminants virus using multi gene sequence data. Vet Microbiol174: 39-49.
- European Food and Safety Authority (2015) Scientific Opinion on peste des petits ruminants. EFSA J 13: 39-85.
- Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, et al. (2010) Global distribution of peste des petitsruminants virus and prospects for improved diagnosis and control. Journal of General Virology91: 2885–2897.
- Libeau G, Diallo A, Parida S (2014) Evolutionary genetics underlying the spread of peste des petits ruminant’s virus. Animal Frontiers4: 14–20.
- Dhar P, Sreenivasa BP, Barrett T, Corteyn M, Singh RP, et al. (2002) Recent epidemiology of peste des petits ruminant’s virus (PPRV) Vet. Microbiol 88: 153-159.
- Kwiatek O, Ali YH, Saeed IK, Khalafalla AI, Mohamed OI, et al. (2011) Asian Lineage of Peste des Petits Ruminants Virus, Africa. Emerg. Infect. Dis 17: 1223-1231.
- Sharawi SSA, Abd El-Rahim IHA (2010) Nucleotide sequencing and phylogenic analysis of fusion (F) epitope for Egyptian Peste-des-petits-ruminant’s virus (PPRV) predicting unique criteria stated as Egypt 2009. Int. J. Virol7: 49–58.
- FAO (Food and Agriculture Organization of the United Nations. Supporting livelihoods and building resilience through Peste des Petits Ruminants (PPR) and small ruminant diseases control. Animal Production and Health Position Paper. 2013
- Cosseddu GM, Pinoni C, Polci A, Sebhatu T, Lelli R, et al. (2013) Characterization of Peste des Petits Ruminants Virus, Eritrea, 2002-2011. Emerg. Infect. Dis19: 160-161.
- Kardjadj M, Ben-Mahdi M, Luka PD (2015a) First serological and molecular evidence of PPRV occurrence in Ghardaïa district, center of Algeria. Trop Anim Health Prod47: 1279-1284.
- Ayari-Fakhfakh E, Ghram A, Bouattour A, Larbi I, Gribâa-Dridi L, et al. (2011) First serological investigation of peste-des-petits-ruminants and Rift Valley fever in Tunisia. The Veterinary J187: 402-404.
- Muniraju M, Munir M, Banyard AC, Ayebazibwe C, Wensman J, et al. (2014) Complete genome sequences of lineage III peste des petits ruminants viruses from the Middle East and East Africa. Genome Announc 2: 01023–1114.
- Wernike K, Eschbaumer M, Breithaupt A, Maltzan J, Wiesner H, et al. (2014) Experimental infection of sheep and goats with a recent isolate of peste des petits ruminants virus from Kurdistan. Vet. Microbiol172: 140-145.
- Libeau G, Prehaud C, Lancelot R, Colas F, Guerre L, et al. (1995) Development of a competitive ELISA for detecting antibodies to the PPRV using recombinant nucleoprotein. Res. Vet. Sci 58: 50-55.
- Al-Majali AM, Hussain NO, Amarin NM, Majok AA (2008) Seroprevalence of, and risk factors for peste des petits ruminants in sheep and goats in Northern Jordan. Prev. Vet. Med85: 1-8.
- Kardjadj M, Kouidri B, Metref D, Luka PD, Ben-Mahdi MH, et al. (2015) Seroprevalence, distribution and risk factor for peste des petits ruminants (PPR) in Algeria. Prev Vet Med122: 205-210. [Crossref]
- FAO (Food and Agriculture Organization of the United Nations). Peste des petits ruminants: an increasing threat to small ruminant production in Africa and Asia. EMPRES Transboundary Animal Disease Bulletin. 2009.
- De Nardi M, Lamin Saleh SM, Batten C, Oura C, Di Nardo A, et al. (2012) First evidence of peste des petits ruminants (PPR) virus circulation in Algeria (Sahrawi territories): outbreak investigation and virus lineage identification. Transbound Emerg Dis59: 214-222. [Crossref]
- Dayhum A, Sharif M, Eldaghayes I, Kammon A, Calistri P, et al. (2017) Sero-prevalence and epidemiology of peste des petits ruminants in Libya. Transbound. Emerg Dis1-7.
- Sghaier S, Cosseddu GM, Ben Hassen S, Hammami S, Haj Ammar H, et al. (2014) Peste des Petits Ruminants Virus, Tunisia, 2012-2013. Emerg Infect Dis20: 2184-2185.
- El Arbi AS, El Mamy AB, Salami H, Isselmou E, Kwiatek O, et al. (2014) Peste des petits ruminants virus, Mauritania. Emerg Infect Dis20: 334-336.
- Kul O, Kabakci N, Atmaca HT, Özkul A (2007) Natural peste des petits ruminants virus infection: novel pathologic findings resembling other Morbillivirus infections. Vet Patho44: 479-486.
- Ozkul A, Akca Y, Alkan F, Barrett T, Karaoglu T, et al. (2002) Prevalence, distribution, and host range of Peste des petits ruminants virus, Turkey. Emerg Infect Dis8: 708-712.
- Albayrak H, Gür S (2010) A serologic investigation for Peste des petits ruminants infection in sheep, cattle and camels (Camelusdromedarius) in Aydin province, West Anatolia. Trop Anim.Health and Prod 42: 151-153.
- Khan HA, Siddique M, Abubakar M, Ashraf M (2008) The detection of antibody against peste des petits ruminants virus in sheep, goats, cattle and buffaloes. Trop Anim Health Prod40: 521-527.
- Singh RP, Saravanan P, Sreenivasa BP, Singh RK, Bandyopadhyay SK, et al. (2004) Prevalence and distribution of Peste des petits ruminants virus infection in small ruminants in India. Revue Scientifiqueet Technique 3: 807-819.
- Abraham G, Sintayehu A, Libeau G, Albina E, Roger F, et al. (2005) Antibody seroprevalences against peste des petits ruminants (PPR) virus in camels, cattle, goats and sheep in Ethiopia. Prev Vet Med70: 51-57.
- Zahur AB, Ullah A, Hussain M, Irshad H, Hameed A, et al. (2011) Sero-epidemiology of peste des petits ruminants (PPR) in Pakistan. Prev Vet Med102: 87-92. [Crossref]
