Influence of Diets Supplemented with Carica Papaya and Chromolaena Odorata Leaf Meals on Performance, Blood Profile and Gut Integrity of Broiler Chickens
Omidiwura BRO1*, Agboola AF1, Omotosho OY1, Mustapha-Olosho JA1
1Department of Animal Science, University of Ibadan, Ibadan, Nigeria.
*Corresponding Author:Omidiwura BRO, Department of Animal Science, University of Ibadan, Ibadan, Nigeria, Tel: +234-708-207-7886; Fax: +234-708-207-7886; E-mail: richardwura@gmail.com
Citation: Omidiwura BRO, Agboola AF, Omotosho OY, Mustapha-Olosho JA (2020) Influence of Diets Supplemented with Carica Papayaand Chromolaena OdorataLeaf Meals on Performance, Blood Profile and Gut Integrity of Broiler Chickens. Medcina Intern 4 (2): 149.
Copyright: © 2020 Omidiwura BRO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: April 09, 2020; Accepted: April 21, 2020; Published: April 23, 2020.
Abstract
The awareness of the negative effects and ban of antibiotics makes it imperative to find effective natural alternatives, such as phytobiotics, to antibiotics to sustain the efficiency of current broiler chicken production. This study was carried out to investigate the effect of Carica papayaand Chromolaena odorataon growth performance, blood profile, gut microbial population, gut morphology and histopathology of broiler chicken. In a 42-day study, 200 one-day old Arbor Acre broilers were weighed and randomly allotted to 5 dietary treatments with 5 replicates having 8 birds in each group. The treatments were basal diet (negative control, NC), basal diet + 0.05% antibiotics (positive control, PC), NC + 3% Carica papaya(CP), NC + 3% Chromolaena odorata (CO) and NC + 1.5% Carica papaya and 1.5% Chromolaena odoratain a completely randomised design. The growth performance indices were measured. On day 42, blood samples were collected, gut microbial population, gut morphology and histopathology were assayed following standard procedure. Data were analyzed using descriptive statistics and ANOVA at α0.05. The result showed that average daily weight gain (g/bird/day) of birds on NC, PC and combination of 1.5% CP + 1.5% CO leaf meals were significantly similar at the finisher phase. The feed intake of birds on NC and PC was higher than those on 3% CO and combination of 1.5% CP + 1.5% CO leaf meals. The dietary treatment had effect on feed conversion ratio in the finisher phase. The highest total Lactobacillus count was observed in 3% CO leaf meal diet, while the antibiotics diet (PC) had the highest Escherichia coli count, and the lowest total Escherichia coli count (3.28 cfu x105) was recorded for birds on combination of 1.5% Carica papaya + 1.5% Chromolaena odorata leaf meals. There were no significance differences in the gut morphological parameters (villus height crypt depth, epithelial wall thickness, epithelial cell) among all the treatments. The histopathology of the gut shows that only those fed 3% CP had eroded villi of the mucosa layer, while all other treatments show normal mucosa layer, moderate inflammatory infiltration of the gland and lamina propia except those fed combination of 1.5% CP + 1.5% CO. In conclusion, either Carica papayaor Chromolaena odorata leaf meal in broiler diet did not significantly affect any of the parameters of interest. However, the combination of the two at 1.5% inclusion in broiler diet had positive effect on beneficial gut microbial population.
Keywords
Leaf Meal, Performance, Carcass Characteristics, Intestinal Microbiota, Blood Metabolites, Broiler Chickens.
Introduction
Antibiotics have been used a long time ago as growth promoters because of its effectiveness in enhancing growth performance for poultry. But Since 2006, the European Union banned antibiotics as growth promoters because of its harmful effects on human health as a result of the drugs toxicity, residual effects and microbial resistance to these antibiotics [1,2]. The National Agency for Food and Drug Administration and Control (NAFDAC) [3] in 2018, also banned the use of antibiotics as feed additives, except when prescribed by a veterinarian. Research has been carried out to look for natural agents with similar beneficial effect as growth promoters, among the natural alternatives are phytogenic feed additives (PFA), PFA they are derived from plants, herbs and spices, and are less toxic. They have been very successful because of their positive effect on growth, improved immune system and reduced stress [4].
Chromolaena odorata(siam weed), – previously known as Eupatorium odorata is a perennial shrub which belongs to the family Asteraceae. The plant contains pharmacologically important phytochemicals such as alkaloids, flavonoids, tannins, saponins, glycosides and phenolic compounds with essential antimicrobial activities [5]. C. odorataleaves are high in crude protein, which is rich in the essential amino acids [6]. Despite the presence of antinutritional factors in C. odorata, researchers have reported the plant as a useful plant in poultry production.
Carica papayais an herbaceous perennial in the family Caricaceaegrown for its edible fruit. It is one of the most popular and economically significant plants in the world [7]. C. papayais a rich source of threes powerful antioxidant vitamin C, vitamin A and vitamin E; minerals; the B vitamins and fibre. The leaf is rich in enzymes like papain and chymopapain, which aid digestion, prevents bloating and other digestive disorders. Carica papaya plays therapeutics role as an antioxidant, anti-inflammatory, analgesic, antimicrobial, anti-ulcer genic and gastro-protectant [8]. Therefore, the present study aimed to evaluate the beneficial effects of Carica papaya(pawpaw) and Chromolaena odorata (siam weed) leaf meals on performance, carcass characteristics, blood metabolites, gut microbial load, gut morphology and histopathology of broiler chickens.
Materials and Methods
Experimental site
The experiment was carried out at the Poultry Unit Teaching and Research farm, University of Ibadan, Ibadan, Oyo state, Nigeria.
Preparation of the test ingredient
Fresh Carica papaya and Chromolaena odorata leaves were obtained from the farm premises. C. papaya and C. odorata leaves were air dried for 3 days. The dried leaves were blended, weighed and mixed with the diets in the right proportion as shown in Tables 1 and 2.
Table 1: Gross composition (g/kg) of broiler starter diets supplemented with Carica papya and Chromolaena odorata leaf meal.
|
INGREDIENTS g/kg |
Negative control (NC) |
Positive control (PC) |
NC + Carica papayaleaf |
NC + Chromolaena odorataleaf |
NC + Carica papaya+ Chromolaena odorata |
|
Corn |
574.00 | 573.50 | 555.00 | 555.00 | 555.00 |
|
Soyabean meal |
360.00 | 360.00 | 349.00 | 349.00 | 349.00 |
|
Fish meal |
30.00 | 30.00 | 30.00 | 30.00 | 30.00 |
|
Soya Oil |
7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
|
Dicalcium phosphate |
13.00 | 13.00 | 13.00 | 13.00 | 13.00 |
|
Broiler premix** |
2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
|
Limestone |
8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
|
Methionine |
2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
|
Lysine |
2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
|
Table Salt |
2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
|
Antibiotics |
0.00 | 0.50 | 0.00 | 0.00 | 0.00 |
|
Pawpaw leaf meal |
0.00 | 0.00 | 30.00 | 0.00 | 15.00 |
|
Siam weed leaf meal |
0.00 | 0.00 | 0.00 | 30.00 | 15.00 |
|
TOTAL |
1000 |
1000 |
1000 |
1000 |
1000 |
|
CALCULATED NUTRIENTS |
|
|
|
|
|
|
Crude protein |
230.36 | 230.31 | 230.89 | 229.44 | 230.17 |
|
Energy ME*, kcal/kg |
3087.72 | 3086.00 | 2992.82 | 2992.88 | 2992.85 |
|
Ether extract |
43.91 | 43.89 | 42.84 | 43.07 | 43.07 |
|
crude fibre |
38.04 | 38.03 | 40.03 | 40.35 | 40.35 |
|
calcium |
8.51 | 8.51 | 8.78 | 8.82 | 8.82 |
|
Total phosphorus |
6.93 | 6.93 | 6.95 | 6.90 | 6.90 |
|
Non-phytate P |
3.54 | 3.54 | 3.56 | 3.67 | 3.62 |
|
Ca:NPP |
2.40 | 2.40 | 2.46 | 2.41 | 2.44 |
*ME- Metabolisable energy. **Vitamin A, 125,500,000 I.U; vitamin D3, 2,500,000 I.U; vitamin E, 40,000mg; vitamin K3, 2,000mg; vitamin B1, 3,000mg; vitamin B2, 5,500mg; niacin, 55,000mg; calcium pantothenate, 11,500; vitamin B6, 5000mg; vitamin B12, 25mg; choline chloride, 500,000mg; folic acid, 1000mg; biotin, 80mg; manganese, 120,000mg; iron, 100,000mg; Zinc, 80,000mg; copper, 8,500mg; iodine, 1,500mg; cobalt, 300mg; Selenium, 120mg; anti-oxidant, 120mg
Table 2: Gross composition (g/kg) of broiler finisher diets fed supplemented with Carica papya and Chromolaena odorata leaf meal.
|
INGREDIENTS g/kg
|
Negative control (NC) |
Positive control (PC) |
NC + Carica papayaleaf |
NC + Chromolaena odorataleaf |
NC + Carica papaya+ Chromolaena odorata |
|
|
Corn |
662.00 | 661.50 | 632.00 | 632.00 | 632.00 | |
|
Soyabean meal |
277.00 | 277.00 | 277.00 | 277.00 | 277.00 | |
|
Fish meal |
25.00 | 25.00 | 25.00 | 25.00 | 25.00 | |
|
Soya Oil |
6.00 | 6.00 | 6.00 | 6.00 | 6.00 | |
|
Dicalcium phosphate |
15.00 | 15.00 | 15.00 | 15.00 | 15.00 | |
|
Broiler premixes** |
2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
|
Limestone |
7.00 | 7.00 | 7.00 | 7.00 | 7.00 | |
|
Methionine |
2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
|
Lysine |
2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
|
Table Salt |
2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
|
Antibiotics |
0.00 | 0.50 | 0.00 | 0.00 | 0.00 | |
|
Pawpaw leaf meal |
0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
|
Siam weed leaf meal |
0.00 | 0.00 | 0.00 | 30.00 | 15.00 | |
|
TOTAL |
1000 |
1000 |
1000 |
1000 |
1000 |
|
|
CALCULATED NUTRIENTS |
|
|
|
|
||
|
Crude protein |
200.68 | 200.63 | 204.73 | 203.28 | 204.00 | |
|
Energy ME*, kcal/kg |
3143.11 | 3141.39 | 3040.14 | 3040.20 | 3040.17 | |
|
Ether Extract |
43.30 | 43.28 | 42.18 | 42.40 | 42.40 | |
|
crude fibre |
34.13 | 34.12 | 36.65 | 36.97 | 36.97 | |
|
Calcium |
8.11 | 8.11 | 8.40 | 8.44 | 8.44 | |
|
Total phosphorus |
6.81 | 6.81 | 6.88 | 6.83 | 6.83 | |
|
Non-phytate P |
3.71 | 3.71 | 3.76 | 3.86 | 3.81 | |
|
Ca:NPP |
2.18 | 2.18 | 2.23 | 2.19 | 2.21 | |
*ME- Metabolisable energy. **Vitamin A, 125,500,000 I.U; vitamin D3, 2,500,000 I.U; vitamin E, 40,000mg; vitamin K3, 2,000mg; vitamin B1, 3,000mg; vitamin B2, 5,500mg; niacin, 55,000mg; calcium pantothenate, 11,500; vitamin B6, 5000mg; vitamin B12, 25mg; choline chloride, 500,000mg; folic acid, 1000mg; biotin, 80mg; manganese, 120,000mg; iron, 100,000mg; Zinc, 80,000mg; copper, 8,500mg; iodine, 1,500mg; cobalt, 300mg; Selenium, 120mg; anti-oxidant, 120mg
Experimental diets and management of birds
Two hundred (200) one-day old Arbor acre broiler chicks with initial average body weight of 41.6 g, sourced from a local hatchery (CHI farm) were randomly allotted to 5 dietary treatments consisting of 5 replicates of 8 birds per replicates in a completely randomized design. The birds were raised from day old to 42 days of age. Treatment 1 was the basal diet (0% leaf meal), Treatment 2 contained basal diet with 0.05% antibiotics, Treatment 3 contained basal diet with 3% Carica papaya leaf meal, Treatment 4 contained basal diet with 3% Chromolaena odorata leaf meal, Treatment 5 contained basal diet with combination 1.5% Carica papaya + 1.5% Chromolaena odorataleaf meal. Experimental diets were given ad libitum and birds had free access to clean water. The experimental diets for both starter and finisher phases were formulated based on the nutritional requirement recommended by the NRC [9], using basically corn-soyabean meal with crude protein (CP) adjusted to 23% and 21% for starter and finisher phases respectively.
Data and sample collection
Performance indices: Performance parameters such as; weight gain, feed intake, feed conversion ratio, feed cost and percentage mortality were calculated. The initial weight was measured on day one of the experiment and subsequently and values were used to calculate weight gain. Feed Intake was calculated as the difference between amount of feed given and left over.
Carcass characteristics: At day 42, one bird per replicate was sacrificed by severing the jugular vein and eviscerated. The primal cuts and organ weights were taken and expressed as percentage of live weight.
Gut microbial load: The ileal digesta samples of one bird per replicate was collected in sterile syringe and taken to laboratory. A ten-fold serial dilution was carried out by transferring 1ml from the gut solution into 9ml of sterile water. This was done to the desired dilution factor of 10-4. 1ml from 10-4 dilution factor was dispensed into respective labeled sterile plates, using a sterile syringe. Afterwards, approximately 15ml of the cooled culture media were poured into each of the plates containing the inoculated samples respectively. To examine the Total heterotrophic count (nutrient agar, incubated aerobically 28h); Lactobacilli (DeMan Rogosa Sharpe agar, incubated anaerobically 48h); Escherichia coli (Eosin methyl blue agar, incubated aerobically 24h); Coliformi (MacConkey, incubated aerobically, 24h). After 48 hours the plates were observed for bacteria and colonies were counted.
Gut Morphology: From the birds sacrificed and eviscerated, the longitudinal sections of the ileal tissue were cut and tissues from each group of birds in replicates were preserved in labeled specimen bottles containing buffered formalin solution and later processed for morphological measurements.
Histopathological Examination: Ileal tissue sections were transfer into specimen bottles containing 10% formalin for preservation. Then normal haematoxylin and eocin standard procedures were performed according to the method of Iji et al., [10]. The tissues were processed and slides prepared were viewed under a microscope and photomicrograph was captured using a motic camera. The photomicrographs were transferred to the computer for the further pathologic reading.
Blood Parameters Measurements
At the end of 42 days, blood samples were collected from the jugular veins of one bird per replicate into two vaccutainer tubes for each broiler chicken, one containing Ethylene Diamine Tetra Acetic Acid (EDTA) for haematological analysis and the other without EDTA for serum analysis. Red blood cell (RBC) and White blood cell (WBC) were determined using Neubauer haemocytometer, after the appropriate dilution. Differential leukocyte counts were performed using the oil immersion objective examination of blood films stained with the modified Romanovsky’s Giemsa stain. Packed cell volume (PCV) was determined as described by Wintrobe [11], using Wintrobe haematocrit method. Haemoglobin concentration was determined by a cyanmethaemoglobin method using Drabkin’s solution as diluent. Platelets were determined by phase microscopy method of Brecher and Cronkite.
Other Serum Biochemical Parameter: The biuret method was utilized in the determination of the total protein fraction while the serum albumin was subjected to the direct colorimetric method as described by Peters et al. [12]. Serum creatinine was determined using the principle of Jaffe reaction as described by Bonsnes and Tausslay [13], while serum urea was determined by the kit (Quinica Clinica Spam), the Uricase method as described by Wootton [14].
Statistical Analysis: Data obtained were analysed by descriptive statistics and Analysis of Variance (ANOVA) procedure of SAS at α0.05. Mean differences were separated using Duncan Multiple Range Test (DMRT).
Results and Discussion
Growth performance of broiler chickens fed Carica papaya and Chromolaena odorataleaf meal supplemented diets
The results of the growth performance of birds fed experimental diets for the starter phase is presented in Table 3. Final weight, average weight gain, average daily weight gain , feed intake, feed intake, feed cost/kg, feed cost/kg/bird and percentage mortality were significantly influenced (p<0.05) by the dietary treatments. The average daily weight gains of birds on control diet and 0.05% antibiotics significantly (p<0.05) higher than those on 3% Carica papaya, 3% Chromolaena odorataand combination of 1.5% Carica papaya+ 1.5% Chromolaena odorataleaf meals. There was no significant difference (p>0.05) in the feed conversion ratio and feed cost/kg weight gain.
The result on growth performance of broiler chicken on experimental diets at the finisher phase is shown in Table 4. Results show that the effect of dietary treatments on Initial weight (b/bird), final weight (g/bird), average weight gain (g/bird), average daily weight gain, feed conversion ratio, feed cost/kg, feed cost/kg weight gain and percentage mortality was significant (p<0.05). The final weight (g/bird) of birds on control diet and 0.05% antibiotics were similar and higher from birds on 3% Carica papaya, 3% Chromolaena odorataand combination of 1.5% Carica papayaand 1.5% Chromolaena odorataleaf meals. Average daily weight gain of birds fed control diet, 0.05% antibiotics and combination of 1.5% Carica papaya+ 1.5% Chromolaena odorataleaf meal was similar. The feed conversion ratio of birds on 3% Carica papaya and combination of 1.5% Carica papayaand 1.5% Chromolaena odorata leaf meals was similar to those on the other experimental diets.
The result revealed that inclusion of Carica papayaand Chromolaena odorataleaf meals in the diet of broilers did not improve the body weight gain of the birds at that inclusion level. This result corroborates the findings of by Haruna and Odunsi [15], who fed broiler chickens crude pawpaw latex. Similarly, Akinmutimi and Akufo [16] reported that increasing dietary inclusion level of Chromolaena odoratado not support good growth performance, carcass quality and organ weight in grower rabbits. The cost of feed per kilogram was higher in the 0.05% antibiotic starter diet (?182.33). This could possibly be as a result of price of antibiotics since other feed additives used were harvested and processed locally.
Birds fed combination of 1.5% Carica papaya+ 1.5% Chromolaena odoratahad the highest average daily weight gain and lowest feed conversion ratio among the leaf meals diets at the finisher phase, although statistically there was no significant difference. This could suggest that Carica papayaand Chromolaena odorataleaf meals had positive combined effect on final weight, average daily weight gain and feed conversion ratio of the birds. Unigwe et al. [17], observed progressive numerical increase in average daily weight gain, average daily feed intake and feed conversion ratio of broiler birds fed sun dried pawpawleaf meal as the level of inclusion increases. Broiler chickens on 3% Chromolaena odorata leaf meal finisher diet had the lowest feed intake but statistically similar to those on 3% Carica papayaand combination of 1.5% Carica papaya+ 1.5% Chromolaena odorata leaf meals. Donkoh et al. [18] concluded that Chromolaena odorata leaf meal addition to broiler diets negatively affects the performance of the bird as shown by decreased feed intake, growth and water consumption. The cost of feed per kilogram weight gain (?562.73) was higher in the 3% Chromolaena odorataand it is similar to those on 3% Carica papayaand 3% Chromolaena odorata leaf meal diets, this could be as a result of higher values of feed conversion ratio recorded. This was in contrast to the findings of Ekenyem et al. [19], which observed least feed cost/kg weight gain of ?52.08 in finisher broilers that had the highest inclusion level of Chromolaena odorata leaf meal.
Table 3: Growth performance of broiler chickens fed Carica papaya and Chromolaena odorataleaf meal supplemented diets (starter phase).
|
Parameters |
T1 |
T2 |
T3 |
T4 |
T5 |
SEM |
P Values |
|
Initial weight (g/bird) |
41.60 | 41.52 | 41.42 | 41.20 | 41.70 | 0.23 | 0.96 |
|
Final weight (g/bird) |
595.38a | 603.92a | 517.58b | 508.54b | 467.28b | 10.13 | 0.0013 |
|
Average weight gain (g/bird) |
553.78a | 562.40a | 476.16b | 467.34b | 425.58b | 10.12 | 0.0013 |
|
Average daily weight gain (g/bird/day) |
26.37a | 26.78a | 22.67b | 22.25b | 20.27b | 0.48 | 0.0013 |
|
Feed intake (g/bird) |
1299.10a | 1225.80a | 1054.10ab | 812.50b | 861.30b | 40.11 | 0.0030 |
|
Daily feed intake (g/bird/day) |
61.86a | 58.37a | 50.20ab | 38.69b | 41.01b | 1.91 | 0.0030 |
|
Feed conversion ratio |
2.35 | 2.17 | 2.36 | 1.74 | 2.09 | 0.13 | 0.54 |
|
Feed cost/kg (?) |
179.14a | 182.33a | 179.13c | 179.13c | 179.13c | 0.0010 | 0.00 |
|
Feed cost (?/kg/bird) |
11.08a | 10.64a | 8.99ab | 6.94b | 7.35b | 0.34 | 0.003 |
|
Feed cost/kg weight gain (?/kg) |
420.45 | 395.84 | 422.67 | 312.03 | 374.69 | 22.55 | 0.54 |
|
Percentage mortality (%) |
13.33b | 10.00b | 16.67a | 3.33c | 3.33c | 2.75 | 0.046 |
ab Means on the same row with different superscript are significantly (p<0.05) different, SEM- Standard Error of Mean, T1- Basal (Control) diet; T2- basal diet + 0.05% antibiotics; T3- Basal diet + 3% Carica papaya leaf meal; T4- Basal diet + 3% Chromolaena odorata leaf meal and T5- Basal diet + 1.5% Carica papaya + 1.5% Chromolaena odorata leaf meals
Table 4: Growth performance of broiler chickens fed Carica papaya and Chromolaena odorataleaf meal supplemented diets (finisher phase).
|
Parameters |
T1 |
T2 |
T3 |
T4 |
T5 |
SEM |
P Values |
|
Initial weight (g/bird) |
595.38a | 603.92a | 517.58b | 508.54b | 467.28b | 1013 | 0.0013 |
|
Final weight (g/bird) |
156.56a | 1600.28a | 1124.54bc | 994.18c | 1230.62b | 30.49 | 0.0001 |
|
Average weight gain (g/bird) |
967.20a | 996.40a | 607.00bc | 485.60c | 763.30ab | 35.51 | 0.0006 |
|
Average daily weight gain (g/bird/day) |
46.06a | 47.45a | 28.90bc | 23.13c | 36.35ab | 1.69 | 0.0006 |
|
Feed intake (g/bird) |
2219.80 | 2086.10 | 1762.60 | 1613.50 | 2346.50 | 150.40 | 0.51 |
|
Daily feed intake (g/bird/day) |
105.70 | 99.34 | 83.93 | 76.83 | 111.74 | 7.16 | 0.51 |
|
Feed conversion ratio |
2.31b | 2.10b | 3.04ab | 3.36a | 2.93ab | 0.14 | 0.046 |
|
Feed cost/kg (N) |
165.37C | 167.91a | 167.52b | 167.52b | 167.52b | 0.010 | 0.00 |
|
Feed cost (N/kg/bird) |
17.48 | 16.68 | 14.06 | 12.87 | 18.72 | 1.20 | 0.53 |
|
Feed cost/kg weight gain (?/kg) |
382.59b | 352.79b | 508.74ab | 562.73a | 490.22ab | 22.85 | 0.043 |
|
Percentage mortality (%) |
28.00a | 3.33c | 3.33c | 10.00b | 29.33a | 5.33 | 0.035 |
abc Means on the same row with different superscript are significantly (p<0.05) different, SEM- Standard Error of Mean, T1- Basal (Control) diet; T2- basal diet + 0.05% antibiotics; T3- Basal diet + 3% Carica papaya leaf meal; T4- Basal diet + 3% Chromolaena odorata leaf meal and T5- Basal diet + 1.5% Carica papaya + 1.5% Chromolaena odorata leaf meals.
Relative carcass and organ weights of broiler chickens fed Carica papaya and Chromolaena odorataleaf meal supplemented diets
The carcass and organ weights of birds are shown in Table 5. Dietary treatment effect on Live weight, breast, wings, drumstick, shank, liver and heart weight (as percentage of live weight) were significant (p<0.05) which contrast findings of Bonsu et al. [20] whostated that the overall carcass characteristics of broilers were not influenced by the inclusion of Chromolaena odorataleaf meal in the diets. No significant influence was observed on weights of head, neck, back, thighs, full gizzard, abdominal fat, spleen, pancreas and bursa of fabricius (as percentage of live weight). The biggest liver was recorded in birds on the combination of Carica papaya and Chromolaena odoratawhile the smallest was observed in birds fed control diet.
Effect of experimental diets on live weight, breast, wings, drumstick, shank, liver and heart weight (expressed as percentage of live weight) was significant. The liver is a major detoxification organ in the body, increase in liver activities leads to increased liver weight. The liver weight was bigger in combination of 1.5% Carica papayaand 1.5% Chromolaena odoratathan control diet this could be due to the presence of anti- nutritional factors in the combination of Carica papayaand Chromolaena odorataleaf meals.
Table 5: Relative carcass and organ characteristics of broiler chickens fed Carica papaya and Chromolaena odorata leaf meal supplemented diets.
|
Parameters (%) |
T1 |
T2 |
T3 |
T4 |
T5 |
SEM |
P Values |
|
Live weight |
1562.56a | 1600.28a | 1124.54bc | 994.18c | 1230.62b | 30.49 | 0.0001 |
|
Head |
2.92 | 2.84 | 3.56 | 3.57 | 3.69 | 0.14 | 0.19 |
|
Neck |
3.63 | 4.88 | 4.53 | 5.54 | 5.48 | 0.29 | 0.25 |
|
Breast |
21.84ab | 25.37a | 23.26ab | 20.45b | 21.49ab | 0.57 | 0.10 |
|
Back |
15.28 | 16.40 | 16.46 | 15.75 | 17.04 | 0.58 | 0.89 |
|
Wings |
7.63b | 9.25ab | 8.92ab | 9.82a | 10.13a | 0.26 | 0.058 |
|
Drumstick |
9.58b | 12.13a | 11.69ab | 10.82ab | 10.44ab | 0.31 | 0.11 |
|
Thighs |
8.83 | 9.56 | 10.02 | 10.03 | 11.73 | 0.55 | 0.56 |
|
Shank |
4.00b | 5.14a | 5.14a | 5.49a | 5.53a | 0.13 | 0.070 |
|
Full Gizzard |
3.63 | 3.71 | 3.96 | 3.49 | 4.00 | 0.15 | 0.78 |
|
Abdominal fat |
0.81 | 0.94 | 0.57 | 0.36 | 0.58 | 0.10 | 0.43 |
|
Liver |
2.15b | 2.52ab | 2.55ab | 2.63ab | 2.77a | 0.077 | 0.18 |
|
Spleen |
0.11 | 0.13 | 0.12 | 0.11 | 0.13 | 0.010 | 0.96 |
|
Heart |
0.49b | 0.64a | 0.57ab | 0.64a | 0.64a | 0.021 | 0.11 |
|
Pancreas |
0.27 | 0.31 | 0.29 | 0.30 | 0.34 | 0.016 | 0.73 |
|
Bursa of fabricius |
0.25 | 0.24 | 0.25 | 0.15 | 0.21 | 0.014 | 0.20 |
abc Means on the same row with different superscript are significantly (p<0.05) different, SEM- Standard Error of Mean, T1- Basal (Control) diet; T2- basal diet + 0.05% antibiotics; T3- Basal diet + 3% Carica papaya leaf meal; T4- Basal diet + 3% Chromolaena odorata leaf meal and T5- Basal diet + 1.5% Carica papaya + 1.5% Chromolaena odorata leaf meals
Gut microbial population of broiler chickens fed Carica papaya and Chromolaena odorata leaf meal supplemented diets
The gut microbial load of broilers fed experimental diets is presented in Table 6. The Total heterotrophic count (THC x105) was significantly higher in the control diet in comparison to other treatments. Total Coliform count (TCC x105) showed similarities (p>0.05) between birds on 3% Carica papayaand 3% Chromolaena odorataleaf meals diets. There was significant difference (p<0.05) in the Total Escherichia colicount across the treatments. Highest Escherichia coli count (23.42 x 105) was recorded in antibiotic diet while least (3.28 x 105) was observed in the combination of Carica papyaand Chromolaena odorataleaf meals. Total Lactobacillus count of all treatments differed significantly (p<0.05) and 0.05% antibiotics had least Total Lactobacillus count while highest TCC counts was recorded in Chromolaena odoratadiet.
The inclusion of 3% Chromolaena odorata leaf meal in the diet increased the gut Lactobacillus count this implies that the presence of Chromolaena odorata in the diet stimulated the proliferation of Lactobacillus. This is in agreement with Jiwuba et al. [21] who posited that Chromolaena odorata leaf meal diets at inclusion levels of 2, 4 and 6% have the ability to stimulate Lactobacillus growth. However, birds on 0.05% antibiotic diet recorded the lowest value of Lactobacillus count. This indicates that antibiotics in the diet had negative effect on beneficial microbes in the gut of broilers. Birds on the combination of Carica papyaand Chromolaena odorataleaf meal diets had lower level of TCC and TEC than those on the individual treatment. The result of this study supported the reports of Omidiwura [22], that methanol-extracted pawpaw leaf can be used as alternatives to synthetic antibiotic in livestock production because it is effective against pathogenic microbial organisms.
Table 6: Gut microbial population of broiler chickens fed Carica papaya and Chromolaena odorata leaf meal supplemented diets.
|
Parameters (Cfu x 105) |
T1 |
T2 |
T3 |
T4 |
T5 |
SEM |
P Values |
|
THC |
73.20a | 23.64d | 27.22c | 48.78b | 14.02e | 0.052 | 0.0001 |
|
TCC |
41.16a | 39.20b | 35.70c | 35.10c | 25.64d | 0.096 | 0.0001 |
|
TEC |
9.20c | 23.42a | 13.66b | 6.24d | 3.28e | 0.050 | 0.0001 |
|
TLC |
17.98c | 3.06e | 14.12d | 27.12a | 21.24b | 0.046 | 0.0001 |
abcde Means on the same row with different superscript are significantly (p<0.05) different, SEM- Standard Error of Mean, THC- Total heterotrophic count, TCC- Total coliform count, TEC- Total Escherichia coli count, TLC- Total Lactobacillus count. T1- Basal (Control) diet; T2- basal diet + 0.05% antibiotics; T3- Basal diet + 3% Carica papaya leaf meal; T4- Basal diet + 3% Chromolaena odorata leaf meal and T5- Basal diet + 1.5% Carica papaya + 1.5% Chromolaena odorata leaf meals
Ileal morphology and histopathology of broilers chicken fed with Carica papaya and Chromolaena odorataleaf meal supplemented diets
Result obtained for ileal morphology (Table 7) shows no significant (P>0.05) difference in villus height, villus width, crypt depth, epithelial wall thickness and epithelial cell. The ileal morphology result shows no significant difference, which agrees with Peric et al., [](2010) that reported no significant difference in the gut morphology of broiler chickens fed phytogenic additives but contradict Wealleans et al. [23] who reported significant difference in the villus height and crypt depth of broiler chickens fed Bacillus probiotics.
Table 7: Ileal morphology of broilers chicken fed with Carica papaya and Chromolaena odorataleaf meal supplemented diets.
|
Parameter |
T1 |
T2 |
T3 |
T4 |
T5 |
SEM |
P-value |
|
Vilus height |
864.3 | 668.6 | 872.3 | 687.1 | 711.2 | 33.058 | 0.2038 |
|
Vilus width |
100.71 | 99.23 | 138.83 | 110.76 | 98.96 | 4.646 | 0.0942 |
|
Crypth depth |
205.16 | 111.4 | 142.01 | 118.37 | 96.8 | 14.81 | 0.2358 |
|
EpitheliumWall thickness |
255.75 | 267.65 | 228.92 | 243.063 | 306.37 | 14.651 | 0.5463 |
|
Epithelium |
28.17 | 26.35 | 31.74 | 27.09 | 27.53 | 1.37 | 0.744 |
Values with different superscript are significantly (p<0.05) different. T1- Basal diet feed (negative control (NC) diet), T2-Basal diet feed +3.0% Neomycin sulphate (positive control (PC) diet), T3-Negative control + 3% Pawpaw leaves, T4-Negative control + 3% Chromolaena odorataleave and T5-Negative control + 1.50% Carica papayaand 1.5% Chromolaena odorata.
As indicated on plates 1-5, for the histopathology of the ileum, it was observed that only 3% Carica papayadiet has eroded villi of the mucosa layer. All other treatments showed moderate inflammatory infiltration of the gland and lamina propia except combination of 1.5% Carica papaya and 1.5% Chromolaena odorata leaf meal diet which shows severe to chronic infiltration of inflammatory cells. The result showed that the inclusion of Carica papaya and Chromolaena odorataleaf meal in the diet of the birds has no effect on the histopathology of the ileum section of the bird as compared to those fed the negative and positive control diet.Agboola and Adenuga [24] reported that higher inclusion level of heat treated jatropha seed cake had a negative effect on the histopathology liver and bursa of fabricus of japanese quails. Figure 1-5.
Figure 1: Plate 1- Birds fed on basal (control diet). Photomicrograph showsnormal mucosa layer with normal villi (white arrow), the glands and lamina propria show moderate infiltration of inflammatory cells (slender arrow), the submucosal layer appear infiltrated by inflammatory cells and also show focal area of lymphocytes aggregate (blue arrow). Muscularis layers appear normal (red arrow).
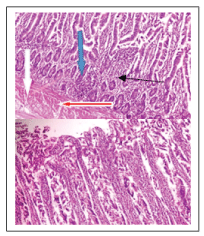
Figure 2: Plate 2- Birds fed on control diet +0.05% Antibiotics (positive control). Photomicrograph shows the submucosal layer appear infiltrated by inflammatory cells and also show focal area of lymphocytes aggregate (blue arrow). Muscularis layers appear normal (red arrow).
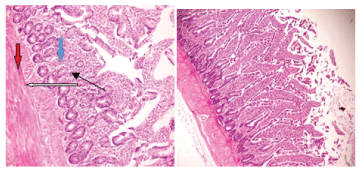
Figure 3: Plate 3- Birds fed on control diet + 3% Carica papaya. Photomicrograph shows mucosa layer with moderately sloughed/ eroded villi (white arrow), the glands and lamina propria show moderate infiltration of inflammatory cells (slender arrow), the submucosal layer appear mildly infiltrated by inflammatory cells (blue arrow). Muscularis layers appear normal (red arrow).
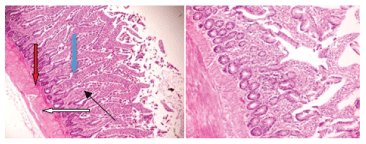
Figure 4: X100 Plate 4- Birds fed on control diet + 3% Chromolaena odorata. Photomicrograph shows moderately normal mucosa layer, the glands and lamina propria show moderate infiltration of inflammatory cells (slender arrow).
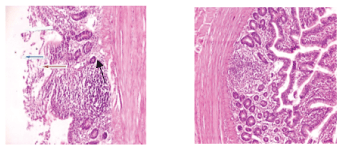
Figure 5: X100 Plate 5- Birds fed on control diet + 1.5% Carica papaya+ 1.5% Chromolaena odorataleaf meals. Photomicrograph shows normal mucosa layer, the glands and lamina propria show severe to chronic infiltration of inflammatory cells (slender arrow).
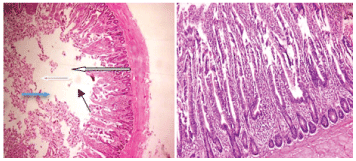
Heamatological indices and serum biochemical parameters of broiler chickens fed Carica papaya and Chromolaena odorata
The result of haematological indices of broiler birds fed experimental diets is presented in Table 8. There was no effect of dietary treatments on all the parameters measured except red blood cell (RBC) count. There was a significant difference between RBC count of birds on 0.05% antibiotics and combination of 1.5% Carica papaya and 1.5% Chromolaena odorata leaf meal diets. The haematological parameters are important indices that reflect the physiological state of the individual animal, the ability to interpret the blood profile in healthy and diseased conditions is one of the primary objectives of haematological studies [25].
Table 8: Haematological indices of broiler chickens fed Carica papayaand Chromolaena odorataleaf meal supplemented diets.
|
Parameters |
T1 |
T2 |
T3 |
T4 |
T5 |
SEM |
P Values |
|
Packed cell volume (%) |
27.80 | 27.00 | 28.6 | 27.20 | 30.00 | 0.58 | 0.49 |
|
Haemoglobin (g/dL) |
9.26 | 8.86 | 9.44 | 9.00 | 9.68 | 0.19 | 0.66 |
|
Red blood cell (x106 uL) |
3.12ab | 2.67b | 3.28ab | 3.04ab | 4.64a | 0.26 | 0.018 |
|
White blood cell (x 107 uL) |
1.91 | 1.73 | 1.59 | 1.90 | 1.64 | 682 | 0.48 |
|
Platelets (x 105 uL) |
2.44 | 1.88 | 2.10 | 2.00 | 1.72 | 1.02 | 0.26 |
|
Lymphocytes (%) |
65.00 | 63.40 | 62.20 | 64.40 | 66.60 | 0.85 | 0.56 |
|
Heterophils (%) |
28.80 | 29.80 | 30.60 | 28.40 | 27.00 | 0.95 | 0.79 |
|
Heterophil/Lymphocytes |
0.45 | 0.48 | 0.49 | 0.45 | 0.41 | 0.02 | 0.75 |
|
Monocytes (%) |
3.60 | 3.60 | 3.80 | 3.20 | 3.20 | 0.27 | 0.94 |
|
Eosinophil (%) |
2.20 | 4.00 | 2.60 | 4.00 | 3.00 | 0.39 | 0.51 |
|
Basophil (%) |
0.20 | 0.20 | 0.40 | 0.00 | 0.20 | 0.085 | 0.70 |
ab Means on the same row with different superscript are significantly (p<0.05) different, SEM- Standard Error of Mean, T1- Basal (Control) diet; T2- basal diet + 0.05% antibiotics; T3- Basal diet + 3% Carica papaya leaf meal; T4- Basal diet + 3% Chromolaena odorata leaf meal and T5- Basal diet + 1.5% Carica papaya + 1.5% Chromolaena odorata leaf meals
Table 9: Serum biochemical parameters of broiler chickens fed Carica papayaand Chromolaena odorataleaf meal supplemented diets.
|
Parameters |
T1 |
T2 |
T3 |
T4 |
T5 |
SEM |
P Values |
|
Total protein (g/dL) |
3.42 | 4.18 | 4.04 | 3.96 | 4.18 | 0.12 | 0.29 |
|
Albumin (g/dL) |
1.87 | 1.78 | 1.77 | 1.89 | 1.94 | 0.55 | 0.84 |
|
Globulin (g/dL) |
1.55b | 2.40a | 2.28a | 2.08ab | 2.24a | 0.087 | 0.047 |
|
ALB/GLO |
1.22a | 0.76b | 0.79b | 0.93b | 0.90b | 0.035 | 0.0033 |
|
Creatinine (mg/dL) |
0.50 | 0.68 | 0.56 | 0.60 | 0.52 | 0.045 | 0.73 |
|
Urea (mg/dL) |
5.31 | 5.38 | 6.37 | 6.29 | 6.06 | 0.22 | 0.39 |
ab Means on the same row with different superscript are significantly (p<0.05) different, SEM- Standard Error of Mean, T1- Basal (Control) diet; T2- basal diet + 0.05% antibiotics; T3- Basal diet + 3% Carica papaya leaf meal; T4- Basal diet + 3% Chromolaena odorata leaf meal and T5- Basal diet + 1.5% Carica papaya + 1.5% Chromolaena odorata leaf meals
The packed cell volume (PCV) and haemoglobin (Hb) of birds within the dietary treatments fell within the normal physiological range of 22.0-35.0 % and 7.0- 13.0 g/dL reported by Jain [26], respectively. This is in agreement with the normal range of PCV and Hb reported by Jiwuba et al. [21] in the study of utilisation of Chromolaena odorata leaf meal by broilers. The red blood cell (RBC) value of birds on combination of 1.5% Carica papaya+ 1.5% Chromolaena odorataleaf meals was outside the range and this could possibly suggest polychytemic condition. The WBC concentration of birds on 3% pawpaw leaf agreed with the findings of Agboola et al. [27] who postulated that sundried pawpaw leaf meal contains some secondary metabolites that enhance the immune system. The lymphocyte, heterophil, Monocyte, eosinophil, basophil percentages and heterophil to lymphocyte (HET/LYM) ratio were within the physiological normal range as reported by Jain [26].
Table 9 shows the results of the analysis of some serum metabolites of birds fed experimental diets. The diets had no significant effect on total protein, albumin, creatinine and urea except globulin and Alb/Glo ratio. Globulin concentration of birds fed 3% Chromolaena odorataleaf meal diet was similar to other diets. The alb/glo ratio of birds on control diet was significantly (p<0.05) higher (1.22) than those on other dietary treatment. The chemistry of serum is normally used for the diagnosis of organ diseases in farm animals and detection of the amount of available protein in the diets [28]. There were no appreciable differences observed in total protein and albumin of birds which contrast the significant differences reported by Jiwuba et al. [21]. The serum urea and creatinine values were within the normal physiological range as reported by Mitruka and Ranwsley [29].
Conclusion
The inclusion of either Carica papaya or Chromolaena odorata leaf meal in broiler diet did not enhance performance, gut morphology and histopathology. The combination of 1.5% Carica papaya and 1.5% Chromolaena odorata in broiler diet had positive effect on the performance indices. The combination could be considered as suitable natural alternatives to synthetic antibiotics in broiler diet.
Recommendation
Carica papaya and Chromolaena odorata at inclusion level of 1.5% + 1.5% is recommended in broiler starter and finisher diets for improved proliferation of beneficial microorganisms. Further studies should be carried out on probably higher inclusion levels of Carica papaya and Chromolaena odoratain broiler diet and also in characterising the beneficial microorganisms.
References
- Mounia M, Nadir A, Omar B (2018) Effects of phytogenic products on gut morpho-histology of broiler chickens. Int. J. Vet. Sci. Res 4: 009-011.
- Fernando U, Biswas D, Allan B, Wilson P, Potter AA (2007) Influence of Campylobacter jejuni fliA, RpoN and Flgk Genes on Colonization of the Chicken Gut. International Journal of Food Microbiology 118: 194-200.
- National Agency for Food and Drug Administration and Control (NAFDAC) (2018) Press release on antimicrobial resistance.
- Youcef M, Letourneau-Montminy MP, Marie-Lou G, Younes C, Gayatri S, et al. (2018) Use of antibiotics in broiler production: Global impact and alternatives. Animal Nutrition4: 170-178.
- Omeke PO, Obi JO, Orabueze NAI, Ike AC (2019) Antibacterial activity of leaf extract of Chromolaena odorata and the effect of its combination with some conventional antibiotics on Pseudomonas aeruginosa isolated from wounds. Journal of Applied Biology & Biotechnology 7: 36-40.
- Igboh MN, Ikewuchi CJ, Ikewuchi CC (2009) Chemical Profile of Chromolaena odorata L. (King and Robinson) Leaves. Pakistan Journal of Nutrition 8: 521-524.
- Ong H, Chua S, Milow P (2011) Ethnomedicinal plants used by the temuan villagers in Kampung jeram kedal, negeri sembilan, Malaysia. Ethnomed Plants 5: 95-100.
- Arshad HR, Yousef HA (2016) Ficus carica and its constituents role in management of diseases. Asian journal of pharmaceutical and clinical research 10.
- NRC (1994) Nutrient Requirement of Domestic Animals. Nutrient Requirement of Poultry. 9th Revised Edition. National Academy Press, Washington DC.
- Iji PA, Saki A, Tivey DR (2001) Body and intestinal growth of broiler chicks on a commercial starter diet. 1. Intestinal weight and mucosal development. Br. Poult. Sci 42: 505- 513.
- Wintrobe MM (1956) Clinical haematology. 4th edition. Kimpton, London. 122.
- Peters T, Biaamonte GT, Doumas BT (1982) Protein (Total protein) in serum urine and cerebrospinal fluid: albumin in serum. In: Selected method of clinical chemistry. Am. Ass. Clin. Chem 9.
- Bonsnes R, Tausslay HH (1945) Colorimetric determination of creatinine by Jaffe reaction. J. Biochem 158: 581- 591.
- Wootton DP (1964) Microanalysis in medical biochemistry, 4th ed. Churchill, London. 86.
- Haruna MA, Odunsi AA (2018) Growth Performance and Carcass Quality of Broiler Chickens Fed Dried Pawpaw (Carica Papaya Linn) Latex. J. World Poult. Res. 8: 31-36.
- Akinmutimi AH, Akufo A (2006) The effect of graded levels of dietary inclusion of siam weed (Chromolaena odorata) leaf meal in grower rabbit diet in tropical environment. Journal of animal and veterinary advances 5: 707-711.
- Unigwe CR, Okorafor UP, Ogbu UM, Nwufoh OC (2014) The Nutritive Profile of Sun-Dried Paw-Paw (Carica Papaya) Leaf Meal and its Effect on the Growth Performance of Broiler Chickens. Int. J. Pure Appl. Sci. Technol 20: 72-78.
- Donkoh A, Atuahene CC, Anang DM, Badu-Botah EK, Boakye KT, et al. (2002) Response of broiler chickens to the dietary inclusion of Chromolaena odorata leaf meal. Journal of Animal and Feed Sciences 11: 309-319.
- Ekenyem BU, Obih TKO, Odo BI, Mba FIA (2010) Performance of finisher broiler chicks fed varying replacement level of Chromolaena odorata leaf for Soya bean meal. Pak. J. Nutr 9: 558-561.
- Bonsu FRK, Kagya-Agyemang JK, Kwenin WKJ, Hope KN, Sekyere FO, et al. (2013) Growth Performance, Haematological Indices and Carcass Characteristics of Broilers Fed Diet Containing Different levels of Chromolaena odorata Leaf Meal. Egerton J. Sci. & Technol 13: 115-125.
- Jiwuba PC, Ogbuewu IP, Dauda E, Azubuike CC (2017) Blood profile and gut microbial load of broilers fed siam weed (Chromolaena odorata) leaf meal in their diets. Agricultura 14: 17-24.
- Omidiwura BRO (2017) Qualitative and quantitative analysis of pawpaw (Carica papaya) leaf extract and its antimicrobial effect in animal production. Nig. J. Anim. Prod 44: 78-83.
- Wealleans AL, Sirukhi M, Egorov IA (2017) Performance, Gut Morphology Effects of a Bacillus Probiotic, Avilamycin and their Combination in Mixed Grains Broiler Diets. Journal British Poultry Science 58: 523-529.
- Agboola AF, Adenuga AA (2015) Performance and organ histopathology of growing Japanese quail fed heat treated jatropha seed cake substituted for Soyabean meal. Trop. Anim. Prod. Invest 18: 1115-2540.
- Khan TA, Zafar F (2005) Haematological study in response to varying doses of estrogen in broiler chicken. International Journal Poultry science 4:748-751.
- Jain NC (1993) Essential of veterinary hematology. Lea & Febiger, Philadelphia.
- Agboola BE, Ologhobo AD, Adejumo IO, GO Adeyemo (2018) Response of Broiler Chickens to Carica papaya and Talinium triangulare Leaf Meal under Normal and Subnormal Diets. Annual Research & Review in Biology 23: 1-7.
- Iyayi EA, Tewe OO (1998) Serum total protein, urea and creatinine levels as indices of quality of cassava diets for pigs. Tropical Veterinary 16: 5967.
- Mitruka BM, Ranwsley HM (1977) Clinical Biochemical and Haematology Reference Values in Normal Exprimental Animals. Masson Pub. USA Inc., N.Y. pp. 21-84.
