Clinical Evaluation of a Novel Nanofiber Wound Matrix for The Treatment of Chronic Lower Extremity Wounds
Matthew J. Regulski1*
1 DPM, Ocean County Foot & Ankle Surgical Associates, 54 Bey Lea Road, Toms River, NJ 08753, USA.
*Corresponding Author: Matthew J. Regulski, DPM, Ocean County Foot & Ankle Surgical Associates, 54 Bey Lea Road, Toms River, NJ 08753, USA, TEL: (732) 505-4500 ; FAX: (732) 505-4500; E-mail:mregulski@comcast.net
Citation: Matthew J. Regulski (2018) Clinical Evaluation of a Novel Nanofiber Wound Matrix for The Treatment of Chronic Lower Extremity Wounds. J Dermatol & Ther 2:111.
Copyright:© 2018 Matthew J. Regulski, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received date: December 10, 2018; Accepted date: December 20, 2018; Published date: December 24, 2018.
Abstract
Chronic non-healing lower extremity wounds, including diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs), present significant challenges for patients and clinicians, and there remains a need for new technologies capable of treating these wounds. The objective of this study was to evaluate clinical experience with a novel nanofiber wound matrix for the treatment of a large, single-center cohort of chronic lower extremity wounds that had failed to heal. Data from 70 patients with 82 chronic wounds (34 DFUs, 34 VLUs, and 14 other chronic wounds) that had received treatment with the nanofiber wound matrix were included in this retrospective study. Wounds had been refractory to previous treatments for an average of 36 weeks. The majority of wounds were healed after 6 - 12 weeks with regular debridement and application of the nanofiber wound matrix: 68 of the 82 wounds (85.0%) achieved complete closure at 12 weeks, with an average time to complete wound closure of 4.8 ± 3.0 weeks. These results demonstrate improvement when compared to other published studies examining various treatment modalities, such as human cellular repair matrix (h-CRM), porcine small intestine submucosa, and a bi-layered cell based product. In particular, a significantly superior closure rate was observed for VLUs treated with the nanofiber wound matrix in this study (90.9% at 12 weeks) compared to those treated with h-CRM in a previous study by the same treating physician (67.6% at 12 weeks). The results of this clinical study suggest that the nanofiber wound matrix may provide significant benefits for the healing of chronic wounds.
Introduction
Chronic lower extremity wounds, including diabetic foot ulcers (DFU) and venous leg ulcers (VLU), are a significant burden for patients and wound care professionals. Chronic wounds do not progress through the standard wound healing process; instead, they persist for an average of 12 to 13 months, stalled in a prolonged inflammatory state [1]. Chronic wounds have a detrimental impact on quality of life and recur in many patients [2]. Standard wound care treatment can include debridement and offloading for DFUs and compression therapy for VLUs; however, the wounds are often difficult to treat and patients may fail to heal with standard options, thus prompting the use of advanced wound therapies.
One promising advanced therapy is the use of wound matrices, which have been designed to play an active role in wound healing. These materials act as a barrier for protection and function as a foundation for cellular ingrowth and angiogenesis, thereby promoting tissue healing and regeneration [1,3-5]. A unique, fully-synthetic nanofabricated wound matrix was recently developed for the local management of cutaneous wounds. Synthetic materials offer the benefit of minimizing risks presented by biologic matrices, including the potential for disease transmission and transplant rejection, while promoting complete healing [6]. The nanofiber wound matrix possesses characteristics conducive to wound healing, including a defined rate of resorption tailored to match the rate of new tissue ingrowth and re-epithelialization; fiber size and organization similar to native extracellular matrix; high porosity capable of supporting cellular infiltration and neovascularization; and fully synthetic biocompatible materials that have excellent handling properties suitable for clinical application [7]. This novel matrix is the first to combine the advantages of fully synthetic construction with the positive attributes of biologic matrices that support biological activity. A pre-clinical study evaluating the use of the nanofiber matrix in a clinically significant porcine wound model found that treatment with the nanofiber wound matrix resulted in faster and more complete wound closure with less inflammation and superior healing quality compared to treatment with a commercially-available xenograft control [8].
The objective of this study was to evaluate clinical experience with the nanofiber wound matrix for the treatment of a large, single-center cohort of chronic wounds.
Methods and Materials
Data were collected retrospectively by the treating physician. Data from patients with chronic wounds that had persisted for at least 4 weeks and were treated using a resorbable nanofiber wound matrix (Restrata™ Wound Matrix, Acera Surgical, St. Louis, MO) were included in this study. Consent for use of de-identified data for medical research or education was provided by all patients.
Prior to initial treatment with the nanofiber wound matrix, all wounds underwent appropriate debridement. The nanofiber wound matrix was prepared for use, including thorough fenestration of the material, and then applied to the wound surface. Following that, a non-adherent dressing was applied.
Weekly follow-up visits were performed. During these visits, patients received additional applications of the wound matrix for up to 12 weeks as deemed appropriate by the physician. Wound area measurements and observation of wound quality were assessed.
Data were collected from the medical records and analyzed by Microsoft Excel. Data collected and analyzed included patient demographics and wound type, size, duration, and disposition. Descriptive statistics were used to summarize patient and wound variables. Kaplan-Meier time-to-event analysis was performed to assess the probability of closure.
Results
Data from 70 patients with 82 wounds treated with the nanofiber wound matrix were included in the study (Table 1). Participants included 43 women (52.4%), and the average age of all patients was 71.5 years (range 25 - 93). The majority of wound types was approximately evenly divided between DFUs (34 wounds, 41.5%) and VLUs (34 wounds, 41.5%). The other wound types that were treated with the nanofiber wound matrix included pressure ulcers, traumatic and postsurgical wounds, non-venous vascular wounds, and necrotic wounds (14 wounds, 17.1%). The average wound size at baseline was 3.35 ± 4.74 cm2 for all wound types. The average size of DFUs was 1.94 ± 2.17 cm2, and the average VLU was 4.79 ± 6.44 cm2. The average wound duration before treatment with the nanofiber wound matrix across all wound types was 36 weeks (range 4 - 132 weeks; DFUs had a longer average wound duration of 42 weeks than VLUs did of 26 weeks). The majority of patients (64 wounds, 78.0%) had failed prior advanced wound therapies, including collagen dressings, acellular matrices, skin grafts, cellular skin substitutes, hyberbaric oxygen, wound vacuum, and topical growth factors.
Table 1: Study demographics and baseline wound characteristics.
| All wounds (n=82) | DFUs (n=34) | VLUs (n=34) | Other woundsa (n=14) | |
|---|---|---|---|---|
| Gender,n (%)b Male Female |
39 (47.5%) 43 (52.4%) |
20 (58.8%) 14 (41.1%) |
14 (41.1%) 20 (58.8%) |
5 (35.7%) 9 (64.2%) |
| Patient age, years Mean ± SD Range |
71.5 ± 11.7 (35-93) |
69.0 ± 13.2 (35-92) |
74.5 ± 11.2 (51-93) |
70.9 ± 6.4 (59-81) |
| Wound age, weeks Mean ± SD Range |
36 ± 32 (4-132) |
42 ± 30 (4-91) |
26 ± 27 (4-87) |
40 ± 46 (5-132) |
| Wound surface area, cm2 Mean ± SD Median, 25%, 75% |
3.35 ± 4.74 1.66, 0.62, 3.98 |
1.94 ± 2.17 0.95, 0.56, 2.86 |
4.79 ± 6.44 2.17, 1.20, 4.58 |
3.26 ± 3.47 2.54, 1.00, 3.98 |
| Previous advanced therapiesC N (%) |
64 (78.0%) |
26 (76.5%) |
27 (79.4%) |
11 (78.6%) |
aOther wound include pressure ulcers, postsurgical wounds, traumatic wounds, non-venous vascular wounds, and necrotic wounds.
bNine patients had two or more separate wounds
cAdvanced therapies include collagen dressings, acellular matrices, skin grafts, cellular skin substitutes, hyberbaric oxygen, wound vacuum, and topical growth factors.
Overall, no adverse events were reported. Gross observations indicated that wounds treated with the nanofiber wound matrix resulted in marked improvement in wound quality and significant reduction in local inflammation (Figure 1).
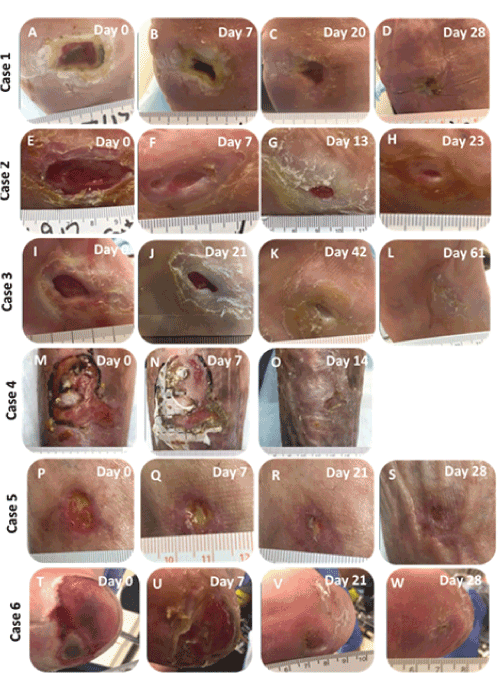
Figure 1:
The wounds demonstrated progressive and sustained wound area reduction over the course of treatment (Table 2). Overall, 53 of the 82 wounds (64.6%) achieved complete wound closure at 6 weeks, and 68 of the 82 wounds (85.0%) achieved complete closure at 12 weeks. The average time to complete wound closure was 4.8 ± 3.0 weeks. At 6 weeks, 61.8% of the DFUs and 67.6% of the VLUs achieved complete closure, and at 12 weeks, 84.8% of DFUs and 90.9% of VLUs achieved complete closure.
Table 2: Wound closure outcomes.
| All wounds (n=82) | DFUs (n=34) | VLUs (n=34) | Other wounds (n=14) | |
|---|---|---|---|---|
| Complete wound closure at 6 weeks Number of wounds, n (%) |
53 (64.6 %) | 21 (61.8 %) | 23 (67.6 %) | 9 (64.3 %) |
| Complete wound closure at 12 weeks Number of wounds, n (%) |
68 (85.0 %) | 28 (84.8 %) | 30 (90.9 %) | 10 (71.4 %) |
Mean ± SD |
48 ± 3.0 | 4.7 ± 2.7 | 5.3 ± 3.4 | 3.7 ± 2.7 |
The proportion of wounds healed by baseline wound size generally showed minimal variation, ranging from 70.0% - 100% overall for all wounds (Figure 2).
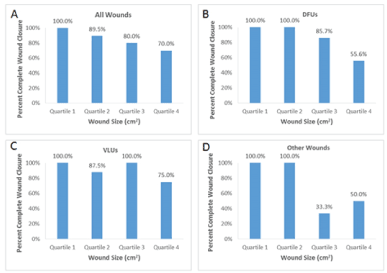
Figure 2:
The probability of wound closure for all wounds at 12 weeks was 85.0% (Figure 3). The probability of closure by 12 weeks was 84.8% for all DFUs, 90.9% for all VLUs, and 71.4% for other wounds.
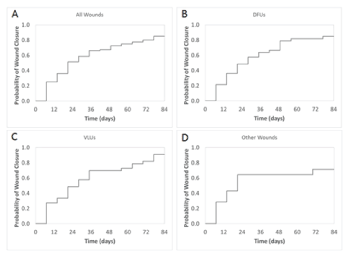
Figure 3:
Discussion and Limitations
This retrospective study was conducted to evaluate the clinical outcomes of chronic wounds treated with a novel synthetic nanofabricated matrix. The study found that application of the nanofiber wound matrix significantly improved clinical outcomes and supported healing in challenging chronic refractory wounds, including DFUs and VLUs. After 6 to 12 weeks, nearly all of the wounds were healed, as evidenced by the fact that 68 of the 82 wounds (85.0%) achieved complete closure at 12 weeks.
These results are encouraging, particularly when compared to another study by the same treating physician using a human cellular repair matrix (h-CRM, Grafix®, Osiris Therapeutics, Inc, Columbia, MD) [9]. In that study, 66 patients with a total of 67 chronic refractory lower extremity wounds, including DFUs (27 wounds, 40.3%), VLUs (34 wounds, 50.7%), and other wounds (6 wounds, 9%), were treated with the h-CRM. The present study includes a comparable number of patients as the h-CRM study, with a higher number of patients in the present study having multiple wounds or wounds other than DFUs or VLUs. Results using the h-CRM revealed complete closure of only 51 out of 67 wounds (76.1%) after 12 weeks and 54 out of 67 wounds (80.6%) after 26 weeks. Although the average wound size at baseline was slightly larger for the h-CRM study (6.65 ± 9.68 cm2 for all wound types) and the average refractory period was slightly longer in duration for the h-CRM study (38 weeks) compared to the present study, the nanofiber wound matrix achieves a significantly higher closure rate in all wounds after 12 weeks (85.0%) compared to the h-CRM (76.1%). Even at 26 weeks, the proportion of healed h-CRM-treated wounds (80.6%) remains lower than the proportion of healed nanofiber wound matrix-treated wounds at 12 weeks (85.0%). Treatment with the nanofiber wound matrix also demonstrates a shorter average time to closure for all wounds (4.8 ± 3.0 weeks) compared to the h-CRM (5.8 ± 2.5 weeks). When evaluated by wound type, the nanofiber wound matrix achieved a comparable closure rate in DFUs (84.8%) compared to the h-CRM (85.2%) at 12 weeks and a superior closure rate in VLUs (90.9% at 12 weeks) compared to the h-CRM at both 12 weeks (67.6%) and 26 weeks (70.6%). A slightly lower closure rate in other wounds (71.7%) was observed in the present study compared to the h-CRM study (83.3%) at 12 weeks, though this may be due to the inclusion of additional wound types (necrotic wounds) that may respond differently.
Other studies have also evaluated various modalities for the treatment of chronic lower extremity wounds with results that are less favorable than the current study. A meta-analysis examining healing rates in DFUs treated with offloading, routine debridement and saline or placebo-gel moistened gauze found that only 24.2% of the wounds were healed after 12 weeks of care [10]. In a randomized clinical trial (RCT) of patients with DFUs, treatment with wound dressings consisting of a nonadherent interface, saline-moistened gauze, dry gauze, and adhesive fixation sheets resulted in 18.3% healed control ulcers at week 12, and treatment with a human fibroblast-derived dermal substitute (Dermagraft®, Organogenesis, Inc., Canton, MA) in addition to the control regimen yielded 30% healed ulcers [11]. A multicenter RCT evaluating healing rates in VLUs found that only 34% of control wounds treated with a nonadherent dressing and a four-layer compression bandaging system and 55% of wounds treated with small intestine submucosa (OASIS® Matrix, Smith & Nephew, Inc., Fort Worth, TX) in addition to the nonadherent dressing and compression therapy exhibited complete healing at 12 weeks [12]. In another multicenter RCT evaluating healing rates in VLUs, results revealed that only 49% of control wounds treated with compression therapy using a self-adherent elastic wrap placed at midstretch tension and 63% of wounds treated with a bi-layered cell based product (Apligraf®, Organogenesis, Inc., Canton, MA) plus compression healed at 6 months [13].
The ability of the nanofiber wound matrix to heal these challenging refractory wounds so effectively may be attributed in part to its proposed mechanism of action, the facilitation of mesenchymal stem cell infiltration and retention and modulation of the inflammatory response. It is well-accepted that blood-borne immunocompetent cells invade the wound site during the inflammatory phase of wound healing, but evidence has revealed that bone marrow-derived stem cells are also recruited into the wound area [14,15]. These mesenchymal stem cells (MSCs) have been shown to engraft to wounded skin, transcribe both collagen types I and III, incorporate and differentiate into skin structures, reconstitute the dermal fibroblast population, improve vasculogenesis, and promote closure of nonhealing wounds [14-16] MSCs have also been shown to have anti-inflammatory effects on the wound healing process; they can respond to the degree of inflammation in the microenvironment by releasing growth factors and cytokines to reduce the inflammatory process, and scaffolds have been found to augment stem cell efficacy [17,18]. The nanofiber wound matrix may play an important role in facilitating MSC homing and retention in the wound site, modulating the inflammatory response, and encouraging wound healing.
The findings from this study indicate that the nanofiber wound matrix is well-suited to support healing of challenging chronic wounds and is well-tolerated by patients. There are limitations to the study, including the lack of a control group and lack of randomization. Additional clinical studies are planned and ongoing to further evaluate the clinical efficacy of the nanofiber wound matrix, investigate its scientific mechanism, and inform the hypotheses of a future randomized controlled trial.
Conclusions
Chronic wounds, particularly those refractory to existing treatment modalities, present significant challenges in the clinical setting. A novel and effective solution for the treatment of chronic wounds in the lower extremity is a nanofiber wound matrix that was shown to reduce local inflammation and heal refractory wounds. More than 80% of nanofiber matrix-treated wounds were healed after 12 weeks of care after being refractory to other treatment options, and the average time to complete wound closure was less than 5 weeks. This nanofiber matrix may provide significant benefits as part of the treatment algorithm for challenging chronic wounds, and future clinical studies are warranted to determine optimal conditions for clinical use.
Disclosure
The author is an advisory board member for Acera Surgical. Product was provided by and manuscript preparation was supported by Acera Surgical.
References
- Frykberg RG, Banks J (2015) Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle) 4: 560-582. [crossref]
- Richmond NA, Maderal AD, Vivas AC (2013) Evidence-based management of common chronic lower extremity ulcers. Dermatol Ther 26: 187-196.
- Shores JT, Gabriel A, Gupta S (2007) Skin substitutes and alternatives: a review. Adv Skin Wound Care 20: 493-508. [crossref]
- James R, Toti US, Laurencin CT, Kumbar SG (2011) Electrospun nanofibrous scaffolds for engineering soft connective tissues. Methods Mol Biol 726: 243-258. [crossref]
- Zhong SP, Zhang YZ, Lim CT (2010) Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2: 510-525.
- Catalano E, Cochis A, Varoni E, Rimondini L, Azzimonti B, et al. (2013) Tissue-engineered skin substitutes: An overview. J Artif Organs 16: 397-403.
- MacEwan MR, MacEwan S, Kovacs TR, Batts J (2017) What makes the optimal wound healing material? A review of current science and introduction of a synthetic nanofabricated wound care scaffold. Cureus 9: e1736.
- MacEwan MR, MacEwan S, Wright AP, Kovacs TR, Batts J, et al. (2017) Comparison of a fully synthetic electrospun matrix to a bi-layered xenograft in healing full thickness cutaneous wounds in a porcine model. Cureus 9: e1614.
- Regulski M, Jacobstein DA, Petranto RD, Migliori VJ, Nair G, et al. (2013) A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage 59: 38-43.
- Margolis DJ, Kantor J, Berlin JA (1999) Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care 22: 692-695.
- Marston WA, Hanft J, Norwood P, Pollak R, Dermagraft Diabetic Foot Ulcer Study G, et al. (2003) The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: Results of a prospective randomized trial. Diabetes Care 26: 1701-1705.
- Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D, et al. (2005) Effectiveness of an extracellular matrix graft (oasis wound matrix) in the treatment of chronic leg ulcers: A randomized clinical trial. J Vasc Surg 41: 837-843.
- Falanga V, Margolis D, Alvarez O, et al. (1998) Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human skin equivalent investigators group. Arch Dermatol 134: 293-300.
- Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P (2003) Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 196: 245-250. [crossref]
- Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, et al. (2004) Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 22: 812-822. [crossref]
- Badiavas EV, Falanga V (2003) Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol 139: 510-516. [crossref]
- Ennis WJ, Sui A, Bartholomew A (2013) Stem Cells and Healing: Impact on Inflammation. Adv Wound Care (New Rochelle) 2: 369-378. [crossref]
- Lam MT, Nauta A, Meyer NP, Wu JC, Longaker MT, et al. (2013) Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing. Tissue Eng Part A 19: 738-747.
