Probiotic Potential of Synbiotic Sweet Product from Orange Peel using Lactobacillus Rhamnosus and Lactobacillus Plantarum
Divya Tandon1*
1Assistant Professor, Microbiology, Shoolini institute of life Sciences and business management, Solan, HP, India.
*Corresponding Author:Divya Tandon, Assistant Professor, Microbiology, Shoolini institute of life Sciences and business management, Solan, HP, India, Tel: 91- 8988186080; Fax: 91- 8988186080; E-mail:
Citation: Divya Tandon (2020) Probiotic Potential of Synbiotic Sweet Product from Orange Peel using Lactobacillus Rhamnosusand Lactobacillus Plantarum.Diabetes Cholest metabol 4: 115.
Copyright: © 2020 Divya Tandon, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 06, 2020; Accepted: February 25, 2020; Published: February 28, 2020.
Abstract
Several studies in recent years have shown the therapeutic benefits derived from the ingestion of probiotic foods. Chocolates are very popular products and hence, can be tested as one of the matrices to carry probiotic. In this context, a study was taken up to develop a synbiotic relationship in orange peel that involves combination of both orange peel and Lactobacillus to be serve in market as health promoting food. In this study we use Lactobacillus plantarum and Lactobacillus rhamnosus, well-recognized probiotic, with orange peel which was converted into powdered form and has prebiotic characteristic, to build a symbiotic relationship. Also inulin was used, a well-recognized prebiotic, as another factor to maintain high viability and survival for both Lactobacillus rhmanosusand Lactobacillus plantraumduringprocedure of processing and storage. Synbiotic sweet was sensory evaluated in microbiology department of college Shoolini Institute of Life Sciences and Business Management, Solan (Himachal Pradesh). The nutritional evaluation was conducted weekly, synchronous with probiotic potential of product.
Keywords:
Keywords: Orange Peel, Probiotic, Synbiotic Sweet, Prebiotic
Introduction
Probiotic defined as live microorganism that when administered in adequate amounts confer a health benefit on the host. Products sold as probiotics include food such as yogurt, dietary supplements and products that are not used orally, such as skin creams. The use of probiotic is not new, although in the early years it was used as food preservation then evolved towards benefits for health, such as reduction of cholesterol and enhances immune system [1] (Pimentel, Madrona, Garcia, and Prudencio, 2015). Prebiotic defined as “A nondigestible food ingredient which beneficially affects the host by selectively stimulating the growth and/or activity of one or limited number of bacteria in the colon and thus improving host health” [2]. Pectin in orange peelats as a prebiotic and encourages the growth of beneficial probiotic bacteria in the intestine (Wolf., 2017). Inulin has a synergic effect on probiotic survival during processing and storage [3]. Prebiotic like inulin can be added easily to orange peel. Lactic acid bacteria are most widely used as probiotics may also improve the balance of intestinal flora if taken alive and in adequate amounts Lactobacillus is most widely recognized as probiotic [4]. Lactobacillus plantarumhas significant antioxidant activities and helps to maintain the intestinal permeability. It can also control the growth of gas producing microorganism in the intestines. Lactobacillus plantarum increase hipocampal brain derived factor which means it have a beneficial role in the treatment of depression [5]. Lactobacillus rhamnosuscan stop allergic reactions to 80% of children [6] and has been shown many other benefits in the prevention of rotavirus diarrhea in children [7]. It has a role in the prevention and treatment of various types of diarrhea both in children and in adults. It reduces the risk of obtaining respiratory tract infection in children. Synbiotics refers to food ingredient or dietary supplements combining probiotics and prebiotics in a form of synergism, hence Synbiotics [8]. Because the word “synbiotics” alludes to synergism, term should be reserved for products in which the prebiotic compounds selectively favour the probiotic organism. A synbiotic product beneficially affects the host in improving the survival and of live microbial dietary supplements in the gastrointestinal tract by selectively stimulating the growth and/ or activating the metabolism of one or a limited number of health promoting bacteria [9]. Orange peel is very useful in producing symbiotic relationship that’s why used in this research. Orange peels are a source of health promoting carbohydrates. Peels also contain healthy polymethoxylated flavones, which are plant pigment compounds, present in all citrus fruits. Orange peel is rich in fiber, which helps to regulate our gastrointestinal tract (Tadin 2018) [10-15].
Materials and Method
Collection of sample and bacterial strains
10kg of orange were collected from the local market of Solan, Himachal Pradesh and then sundried until the moisture content would be 10%. The strains used were Lactobacillus rhamnosusand Lactobacillus plantarumprocured from the Department of Microbiology, SILB, and SOLAN. L.rhmanosusand L.plantarumindividually were released in 9ml of Man, Rogosa and Sharpe (MRS) broth, and incubated at 37? for 24 h [16-21]. After the incubation of 24h L.rhamnosusand L.plantarumwere incubated individually in skim milk broth (12% w/v) at 37? for 24h.
Ingredients for sweet production
Sweet were produced by using the following commercial ingredients: Orange peels, condensed sweeten milk (10%), inulin (10%) and cooking chocolate (50%) [22-24].
Synbiotic sweet production
Take solid ingredients (20%) orange peel and mix them with (10%) inulin for 3min. Liquid ingredients 10% starter of L.rhmanosusand L.plantarumadded individually and 10% condensed milk mix them well for 5min. Cooking chocolate were purchased from the local market of Solan, Himachal Pradesh and added after melting it in water bath at temperature 60? until it became runny and easy to stirring [25-27]. Then chocolate was taken out and left for cooling down to 4? then added to mixture. Spread the mixture with the desired thickness then kept it in refrigerator for 10min [28-31]. Cover the chocolate with aluminum foil and kept it in refrigerator at 4°C (Bashar et al., 2015).
Synbiotic sweet production
Take solid ingredients (20%) orange peel and mix them with (10%) inulin for 3min. Liquid ingredients 10% starter of L.rhmanosusand L.plantarumadded individually and 10% condensed milk mix them well for 5min. Cooking chocolate were purchased from the local market of Solan, Himachal Pradesh and added after melting it in water bath at temperature 60? until it became runny and easy to stirring [25-27]. Then chocolate was taken out and left for cooling down to 4? then added to mixture. Spread the mixture with the desired thickness then kept it in refrigerator for 10min [28-31]. Cover the chocolate with aluminum foil and kept it in refrigerator at 4°C (Bashar et al., 2015).
Storage Studies
The efficiency of the symbiotic sweet in maintaining an appropriate viable number of probiotics were acknowledged after 2 months of storage in refrigerator at temperature 4°C (Bashar et al., 2015) [37,38].
Sensory Evaluation
The sensory evaluations of product were done on the basis of 5 point hedonic scale. The sensory evaluations were conducted weekly, synchronous with microbiological assays [39]. Five parameters were used: Colour, Appearance, Texture, Taste and Odor with scale from 1 to 10 for all (Batista et al., 2017).
Nutritional Evaluation
• Sugar test by DNSA method (Miller 1959).
• Protein test by Lowry’s method (Lowry 1951).
• Phenols test by ferric chloride test (Evan 1989).
• Moisture (Initial amount of orange peel- Dried amount of orange peel (Fischer 1935).
Probiotic potential
Acid Tolerance (Conway 1987): Product were inoculated in MRS broth and kept for overnight incubation at 37°C. Each product was adjusted to 3.0, 2.5, and 2.0 with 5N HCl and incubated at 37°C for 24 hours. Bacterial growth was monitored for measuring absorbance with a spectrophotometer at 620nm [40].
Bile Tolerance [10]
Product were inoculated in MRS broth and kept for overnight incubation at 37°C. Saturated bile solutions of different bile salt concentration were prepared separately by dissolving powdered bile extract. The cultures were incubated at 37°C for 24hours. Bacterial growth was monitored by measuring absorbance with a spectrophotometer at 540nm.
Antibiotic susceptibility test (Kirby et al., 1950)
The Muller hinton agar (MHA) plate surface is inoculated by spreading volume of the microbial inoculum of Lactobacillus plantarumand Lactobacillus rhamnosusover the entire agar surface. Then, a hole with a diameter of 6 to 8mm is punched aseptically with sterile tip, and a volume (20-100µl) of the antibiotics (Tetracycline and Streptomycin) at desired concentration is introduced into the well [41]. Then, the agar plates were incubated for 24-48hrs at 37°C. The zone of incubation is measured by callipers in millimetres.
Results and Discussion
Microbiological analysis: Microbiological quality of the chocolate was evaluated based on viable Lactobacillus count, yeast and mold count and coliform count.
Lactobacillus count: The main purpose of preparing the chocolate was to supply maximum possible viable probiotic cells to the consumer’s table 3 shows that Lactobacillus count. The average count of Lactobacillus plantarumwas 3.74×106 cfu/g Lactobacillus rhamnosuswas 3.73 ×106 cfu/g and the combination of both Lactobacillus plantarumand Lactobacillus rhamnosus3.76 ×106 cfu/g.
Coliform count: To access the hygienic quality of the chocolate coliform count was done on MacConkey agar plates. It was observed that coliforms were absent in all the samples [42]. This was expected as the chocolate were made in laboratory with hygienic care and stored at very low temperature of 4°C.
Yeast and mold count: Yeast and mold count in all the chocolate sample was estimated by pouring first dilution of chocolate in PDA agar and incubating the plates at 25°C for 3-5 days. The yeast and mold count was nil in the samples. As the chocolate were made in the laboratory under controlled conditions and stored at low temperature 4°C the count was nil in first dilution of the chocolate sample [43].
Storage Studies
Changes in microbiological quality during storage:
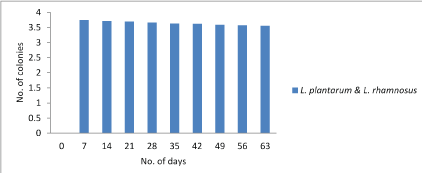
Lactobacilli count: The main objective of the study was to estimate the viability of probiotic culture in the chocolate. During storage for 7th days at 4°C the count reduced to 3.72×106, 3.72×106, 3.74×106 cfu/g for Lactobacillus plantarum, Lactobacillus rhamnosus and combination of both Lactobacillus plantarum and Lactobacillus rhamnosusrespectively. However, on 63th day the Lactobacillus count was not decreased below 3.45×106, 3.50×106 and 3.55×106 for Lactobacillus plantarum, Lactobacillus rhamnosusand combination of both Lactobacillus plantarumand Lactobacillus rhamnosusrespectively. Hence, it was concluded that the count doesn’t fall below 1000 which is sufficient dosage for any food to call as probiotic. Figure 1-3.
Figure 1: Graphical representation of change in Lactobacillus plantarumcount.
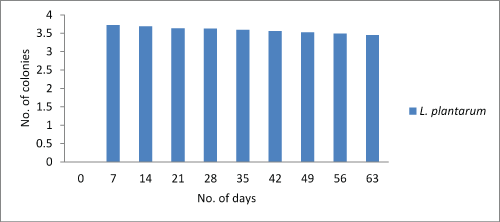
Figure 2: Graphical representation of change in Lactobacillus rhamnosuscount.
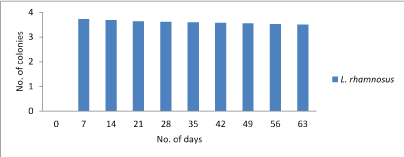
Figure 3: Graphical representation of change in Lactobacillus plantarumand Lactobacillus rhamnosuscount.
Coliform count: To access the hygienic quality of the chocolate coliform count was done on MacConkey agar plates. It was observed that coliforms was absent during storage period (0-63 days) in the first dilution. This was expected as the chocolate were made in laboratory with hygienic care [44].
Yeast and mold count: Yeast and mold count in all the chocolate samples during storage period 0, 7, 14, 21, 28, 35, 42, 49, 56, 63days was estimated by pouring first dilution in PDA agar. The count was nil in first dilution of the samples.
Sensory Evaluation: Sensory score based on 9 point hedonic scale of 3 types of synbiotic chocolate. The average score for all the three synbiotic chocolate was different for all five parameters i.e colour, appearance, taste, texture and odor. As shown in Table 7 the colour score ranges from 7 to 3. The appearance score ranges from 6 to 3. The taste score ranges from 7 to 4. The texture score ranges from 4 to 3 and the odor score ranges from 6 to 3 for all three synbiotic chocolate. Overall the entire three products were liked by the judges [45].
Nutrional evaluation
Sugar test: The concentration of reducing sugar showed decrease in the value. The concentration of reducing sugars was decreasing after 28 days of storage. The presence of reducing ends in the chocolate and the measurements of the concentration of reducing ends produced valuable information about the samples to be analysed. Reducing sugar have gained considerable importance which lead to a growing number of methods for their assay. The level of reducing sugar helps determine the quality of chocolate [46].
Anamaria et al., (2012) showed that significantly higher amount of reducing sugars of 5.67 were found in T5, whereas the lowest content of 0.92 was observed in T1 chocolate. Figure 4-6.
Figure 4: Graphical representation of changes in sugar content of Lactobacillus plantarum.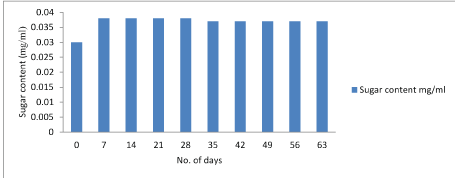
Figure 5: Graphical representation of changes in sugar content of Lactobacillus rhamnosus.
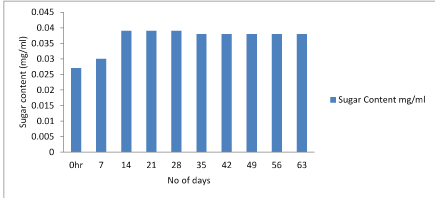
Figure 6: Graphical representation of changes in sugar content of Lactobacillus plantarum & Lactobacillus rhamnosus.
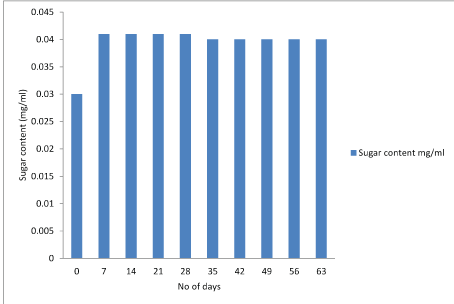
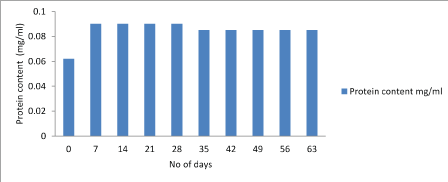
Protein test: The lowry protein assay is a biochemical assay for determining the total level of protein in the product. The total protein concentration is exhibited by a color change of the sample solution. Initially the concentration of protein was high and after storage of 28 days it starts decreasing. Figure 7-9.
Figure 7: Graphical representation of changes in protein content of Lactobacillus plantarum.
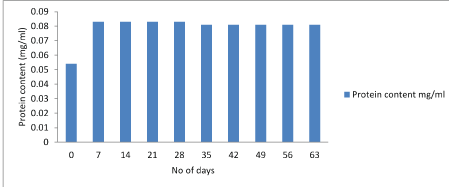
Figure 8: Graphical representation of changes in protein content of Lactobacillus rhamnosus.
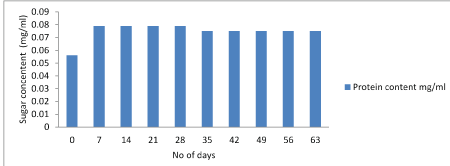
Figure 9: Graphical representation of changes in protein content of Lactobacillus plantarumand Lactobacillus rhamnosus.
Phenol test: Synbiotic chocolate using Lactobacillus plantarum and Lactobacillus rhamnosus gives positive phenol test.
Moisture
Estimation of moisture is used to check the amount water content present in the orange peel. Moisture=Initial amount of the orange peel- dried amount of the orange peel. Moisture= 65gm – 20gm = 45gm
Probiotic potential
Acid tolerance: All three Lactobacillus strains i.e. Lactobacillus plantarum, Lactobacillus rhamnosusand combination of both Lactobacillus plantarumand Lactobacillus rhamnosuswere assayed for acid tolerance.
All the three isolates from the symbiotic chocolate show maximum tolerance at pH 2 and minimum tolerance. These results indicate that the strains were able to tolerate acidic conditions and survive at pH 2.
Bile tolerance: All three Lactobacillus strains i.e. Lactobacillus plantarum, Lactobacillus rhamnosusand combination of both Lactobacillus plantarumand Lactobacillus rhamnosuswere assayed for bile tolerance [47,48].
All three isolates from the synbiotic chocolate show maximum tolerance at 2% and minimum tolerance at 4%. Although the bile concentration of the human gastrointestinal tract varies, the mean intestinal bile concentration believed to be 0.3% (w/v).
Antibiotic susceptible: Resistance was defined according to the standard disc diffusion method by using antibiotics discs of Streptomycin, Tetracycline. All isolates L. plantarumand L. rhamnosuswere sensitive to two of them. The zone of inhibition was measured by callipers in milimeters. All the isolates L. plantarum, L. rhamnosusshowed minimum zone of inhibition to antibiotic Streptomycin. Maximum zone of inhibition of Tetracycline was seen in all three isolates i.e. Lactobacillus plantarum, Lactobacillus rhamnosusand combination of both Lactobacillus plantarum and Lactobacillus rhamnosus.
References
- Food and Agriculture Organization US (FAO) / World Health Organization (WHO). Report on Joint FAO/WHO (2001) Expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria.
- Gibson GR, Roberfroid MB (1995) Dietary modulation of the colonic microbiota: Introducing the concept of prebiotics. Journal of Nutrition 125: 1401-1412.
- De Souza-Oliveira RB, Perego P, Converti A, Oiliveira MN (2009) The effect of inulin as prebiotic on the production of probiotic fiber-enriched fermented milk. International Journal of Dairy Technology62: 195 - 203.
- Espinoza-Rivera Y, Gallardo-Navarro Y (2010) Non-dairy probiotic products. Food Microbiology27: 1-11.
- Bested AC, Logan AC, Selhub ME (2013) Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part I - autointoxication revisited. Gut Pathog5: 5.
- Scott S (2015) Peanut allergies: Australian study into probiotics offers hope for possible cure.
- Canaani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo ML, et al. (2007) Isolate lactic acid bacteria from non-Malaysian chicken broiler chickens (Gallus Gallus) with a potential probiotic intestinal feeding broiler chickens. IIUM Engineering Journal12: 4.
- Pandey KR, Naik SR, Vakil BV (2015) Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol52: 7577-7587. [Crossref]
- Cencic A, Chingwaru W (2010) The role of functional foods, nutraceuticals and food supplements in intestinal health. 2: 611-625.
- Gilliland SE, Speck MJ (1977) Deconjugation of bile acids by intestine Lactobacilli. Applied Microbiology33: 15-18.
- Abrahamsson TR, Sinkiewicz G, Jakobsson T, Fredrikson M, Bjorksten B, et al. (2009) Probiotic Lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J Pediatr Gastroenterol Nutr49: 349-354.
- Alegro LCA, Alegro JHA, Cardarelli HR, Chiu M, Saad SMI, et al. (2007) Potentially probiotic and synbiotic chocolate mousse. LWT Food Science and Technology 40: 669-675.
- Alison C, Alan C, Eva M (2013) Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part I – autointoxication revisited. Gut Pathogens5: 5.
- Bandyopadhyay B, Narayan Mandal C (2014) Probiotics, Prebiotics and Synbiotics -In Health Improvement by Modulating Gut Microbiota: The Concept Revisited. International Journal of Current Microbiology and Applied Biosciences 3: 410-420.
- Bauer AW, Kirby WM, Sherris JC, Turck M (1996) Antibiotic susceptibility testing by a standardized single disk method. J Clin Pathol45: 493-496.
- Bixquert Jiménez M Selective (2009) Colorectal cancer screening in average-risk populations 101: 821-829.
- Boylston TD, Vinderola CG, Ghoddusi HB, Reinheimer JA (2004) Incorporation of bifidobacteria into cheeses: challenges and rewards. International Dairy Journal 14: 375–387.
- Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13: 701-712. [Crossref]
- Chan HK, Sahadeva RPK, Leong SF (2011) Survival of commercial probiotic strains to pH and bile. J Int Food Res 18: 1515-1522.
- Chen P, Cheng Y, Deng S, Lin X, Huang G, et al. (2010) Utilization of Orange peels residues. Int J Agric & Biol Eng. 3(4): 1-18.
- Champagne CP, Laing RR, Roy D, Mafu AA, Griffiths MW (1994) Psychrotrophs in dairy products: their effects and their control. Crit Rev Food Sci Nutr 34: 1-30. [Crossref]
- Del Piane, Ballare M, Monitino M, Orsello F, Garello M, et al. (2004) Clinical experience with probiotics in the elderly on total enteral nutrition. J Clin Gastroenterol 38: S111-S4 [PubMed]
- Desai A (2008) Strain identification, viability and probiotic properties of Lactobacillus casie, Thesis. Australia: School of Biomedical and health sciences, Victoria University, Werribee campus Victoria. 48-53.
- Di Criscio T, Fratianni A, Mignogna R, Cinquanta L, Coppola R, et al. (2010) Production of functional probiotic, prebiotic, and synbiotic ice creams. J Dairy Sci93: 4555-4564. [Crossref]
- EI-Mabrok ASW, Hassan Z, Mokhtar AM, Hussain KMA, Kahar FKSBA, et al. (2012) Screening of lactic acid bacteria as biocontrol against colletotrichum capsid on chilli Bangi. Res J Appl Sci 7: 466-473.
- Falk P, Hoskins IC, Larson (1998) Bacteria of the human intestinal micobiota produce glycosidases specific for lacto-series glycosphingolipids. J Biochem 108: 466-474.
- Flint HJ, Dunacan SH, Scott KP, Louis P (2007) Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol9: 1101-1111.
- Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66: 365-378. [Crossref]
- Garaiova I, Muchova J, Nagyova Z, Wang D, Li JV, et al. (2015) Probiotics and vitamin C for the prevention of respiratory tract infections in children attending preschool: a randomized controlled pilot study. European Journal of Clinical Nutrition 69: 373–379.
- Gibson GR, Fuller R (2000) Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J Nutr 130: 391S-395S. [Crossref]
- Goldin BR, Gorbach SL (2008) Probiotics for humans. In the scientific Basis ed. Fuller R 355-376.
- Kelly G (2008) Inulin-type prebiotics--a review: part 1. Altern Med Rev13: 315-329. [Crossref]
- Grimoud J, Durand H, Courtin C, Monsan P, Ouarné F, et al. (2010) In vitro screening of probiotic lactic acid bacteria and prebiotic glucooligosaccharides to select effective synbiotics. Anaerobe 16: 493-500. [Crossref]
- Guarner F, Sanders ME, Gibson G, Klaenhammer T, Cabana M, et al. (2011) Probiotic and prebiotic claims in Europe: seeking a clear roadmap. Br J Nutr 106: 1765-1767. [Crossref]
- Hassanzadazar H, Ehsani A, Mardani K, Hesari J (2012) Investigation of antibacterial, acid and bile tolerance properties of lactobacilli isolated from Koozeh cheese. Vet Res Forum3: 181-185. [Crossref]
- Hawaz E (2014) Isolation and identification of probiotic lactic acid bacteria from curd and in vitro evaluation of its growth inhibition activities against pathogenic bacteria. Afr J Microbiol Res 4: 1919-425.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, et al. (2014). “Expert consensus document. The international Scientific Associated for Probiotic and Prebiotics consensus statement on the scope and appropriate use of the term probiotic”. Nature Reveiws. Gastroenterology and Hepatology 11: 506-14.
- Hoffman FA, Hiembach JT (2008) Executive summary: scientific and regulatory challenges of development of probiotics as food and drugs 46: 553-557.
- Islam T, Sabrin F, Islam E, Billah M, Islam Didarul KM, et al. (2012) Analysis of antimicrobial activity of Lactobacillus paracasei-1 isolated from yogurt. J Microbiol Biotechnol Food Sci 7: 80-89.
- Jargensen JH, Turnidge JD, Murray PR, Baron EJ, Jorgensen JH, et al. (2007) Antibacterial susceptibility tests: dilutions and disk diffusion method, Manual of clinical microbiology. Washington, DC American Society for Microbiology 1152-1172
- Jay JM (1996) Modern Food Microbiology. 5th Ed. New York: Van No Strand Reinhold.
- Kaur IP, Chopra K, Saini A (2002) Probioics: potential pharmaceutical applications. European J PharmaSci 15: 1-9.
- Konstantinov SR, Kuipers EJ, Peppelenbosch MP (2013) Functional genomic analyses of the gut microbiota for CRC screening. Nature Reviews Gastroenterology and Hepatology 10: 741-745.
- Krasekoopt W, Bhandaria H, Deeth H (2003) Evaluation of encapsulation techniques of probiotics for yogurt. J Int. Diary13: 3-13.
- Lee YK, Salminen S (1995) The coming of age of probiotics. J Trends Food Sci Technol. (6): 241-246.
- Leroy F, De Vuyst L (2004) Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol15: 67-78.
- Magdalena Araya, Ctherine Stanton, Lorenzo Morelli, Greogor Reid, Maya Pinerio, et al. (2006) “Probiotic in food: health and nutritional properties and guidelines for evaluation”. Combined Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic acid bacteria, Cordoba, Argentina (2001), and Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada. 1-50.
- Rivera-Espinoza Y, Gallardo-Navarro Y (2010) Non-dairy probiotic products. Food Microbiol27: 1-11. [Crossref]
