Biological Potent Heteroaryl Ketone Schiff Base and Thorium(IV) Complexes of 2-Benzoaminothiazole
Neelima1*
1 Department of Chemistry Sanskrity University, Mathura-281401, Uttar Pradesn, India.
*Corresponding Author: Neelima, Department of Chemistry Sanskrity University, Mathura-281401, Uttar Pradesn, India, TEL:+91 7500723300 ; FAX:+91 7500723300;E-mail:neelima2010.669@rediffmail.com
Citation: Neelima (2018) Biological Potent Heteroaryl Ketone Schiff Base and Thorium(IV) Complexes of 2-Benzoaminothiazole. Arch Mol Med & Gen 2:107.
Copyright: : © 2018 Neelima, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received date: November 17, 2018; Accepted date: November 27, 2018; Published date: December 01, 2018
Abstract
Metal-based antioxidants has received effort in order to identify the compounds having high free radical scavenging capacity related to various disorders and diseases associated with oxidative damage due to reactive oxygen species (ROS). Two mononuclear Th(IV) complexes were derived from 2,3–dihydro–1H–indolo[2,3–b]phenazine–4(5H)–ylidene)benzothiazole–2–amine (L1), and 3–(ethoxymethylene)–(2,3–hihydro–1H–indolo[2,3–b]phenazine–4(5H)–ylidene)benzothiazole–2–amine (L2) with properties of pharmacologically interest. The compounds were characterized by elemental analyses, molar conductance, magnetic susceptibility measurements, FTIR, UV–Vis, 1H NMR, TGA, XRD, SEM studies. In both complexes 2:1 ligand-to-metal ratio has been observed.
Keywords
antioxidant, metal complex, metal organic frame work etc.
Introduction
Oxidative stress, causehigh levels of protein carbonyl content (CO) groups have been observed in diseases such as Alzheimer’s disease (AD), rheumatoid arthritis, diabetes, sepsis, chronic renal failure, and respiratory distress syndrome [1]. Schiff base are typically synthesized from an aldehyde or ketone with a primary amine and a wide variety of steric and electronic features into their structure [2-6]. Green chemistry, also known as sustainable chemistry, is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances [7-9]. Metals are indispensable for life, as they are involved in many fundamental biological processes, including osmotic regulation, catalysis, metabolism, bio mineralization, and signal transduction. Metal-based compounds have an increasing importance in most prominent biomedical applications, as they offer the possibility of exploring a higher variety of structural motifs and reactivity patterns when compared with purely organic molecules [10]. Thorium(IV)) metal ions have atomic radii 1.17Å and have high positive charge, so that these metals can easily achieve high coordination number. Thorium show complexation tendency in its +4 oxidation easily [7]. Complexes of Th(IV) formed with humates, indicating that Th(IV) preferentially binds to carboxylate groups, which in turn allow for predicting the per mass thorium binding capacity of natural organic matter. The oxidative damage caused by ROS on lipids, proteins, and nucleic acids plays a significant role in the development of life limiting chronic diseases such as cancer, hypertension, cardiac infarction, arteriosclerosis, rheumatism, and cataracts [11,12].The activity is usually increased by complexation, therefore to understand the properties of both ligands and metal can lead to the synthesis of highly active compounds.C. Prabhakaran and co-workers introduced [13] Ni(II) complex of Schiff base showed has an excellent DPPH radical-scavenging effect higher than that of well-known ascorbic acid. Li et al. [14] explained in vitro antioxidant activities of ligands and Sm, La, Nd, and Yb complexes. The results of in vitro SOD and H2O2 scavenging activities of chromone- 3-carbaldehyde-(isonicotinoyl) hydrazone) ligands and their respective metal complexes showed that ligands and their complexes more effective than ascorbic acid. Neelima et al. [15] investigated complex of Th(IV) complexes and their ligands derived from 2,3–dihydro–1H–indolo[2,3–b]phenazine–4(5H)–ylidene)thiazole–2–amine (L1), and 3–(ethoxymethylene)–(2,3–hihydro–1H–indolo[2,3–b]phenazine–4(5H)–ylidene)thiazole–2–amine (L2) have shown antioxidant activities by DPPH and H2O2 scavenging activities methods. Their perceptive binding ability for Th(IV) endows them with potential to reduce the generation of ROS. In this perspective, this finding is an important influence towards mechanistic aspects associated with the promising pharmacological applications and these studies may help to predict the new and novel therapeutic strategies for the treatment of human oxidative-stress based disorders such as muscular dystrophy (MD), Parkinson’s diseases, cancer, and arthritis Alzheimer’s. Additionally, we also explore excellent antimicrobial activities of ligands and metal complexes by micro broth dilution method.
Materials and Methods
Materials
All reagents were obtained chemically pure and were of analytical reagent grade. The solvents were dried and distilled before used. Thorium metal salt and 2-benzoaminothiazole were purchased from Sigma Aldrich and used without further purification. Antimicrobial strains were tested against two-gram positive StaplococousaureusATCC 6538 and Bacillus subtilisATCC 6633, gram negative bacteria Escherechia coli ATCC 25922 and yeast like fungi Candida tropicalisATCC 13803, and Aspergilusniger MTCC 282.
Characterization
Melting points were recorded in capillary tubes on a digital Buchi apparatus and are uncorrected. Elemental analyses were performed using a Perkin Elmer 2400 series II instrument and metal content was determined by gravimetrically as metal oxide. Fourier transform infrared (FTIR) spectra were recorded on a Bruker Vertex 70 FTIR spectrometer by using KBr pallets in the transmission mode, in the range 400–4000 cm-1 at room temperature with a resolution of 2 cm-1. The 1H NMR spectra were recorded on a Bruker Advance II spectrometer and measured at 400 MHz. The magnetic susceptibilities measurements on powder form of the complexes with magnetic susceptibility balance (Mk1) of Sherwood Scientific Corporation, UK using MgSO4 as a celebrant at room temperature. Molar ionic conductance of the complexes was determined in DMSO using a solution of about 10-3 M concentration on ESICO model-1601 microprocessor based conductivity meter with a dip type cell with platinized electrode. G3 Heidolph rotary evaporator was used for the evaporation of solvents. Microwave assisted procedure was carried out in microwave synthesizer CEM-Discover- 925105 of Lab India. TGA studies were carried out by using Perkin Elmer TGA-4000. Data of the scavenging activity were expressed as the mean ± SEM from three independent experiments. One-way ANOVA and Dunnett’s multiple comparisons test was performed using graph pad prism (Version 5.01) software for significance test, using a p value ≤ 0.05.
Procedure
Syntheses of Schiff base ligands
Synthesis of ligand L1
Ligand (L1) was prepared in ethyl alcohol (2 ml) by the condensation with 2-aminobnzothiazole and ketone (K1) [16] in 1:1 equimolar ratio along with catalytic amount of hydrochloric acid, and the resulting mixture was irradiated under microwave for 4-5 min. Allowed to cool to ambient temperature, acidiï¬ed with acetic acid (pH 5.7) and then refluxed for 1 min. The extra solvent was removed by rotary evaporator. Brown precipitate was collected by ï¬ltration and drying (Scheme 1). The product was recrystallized from the same solvent and dried.
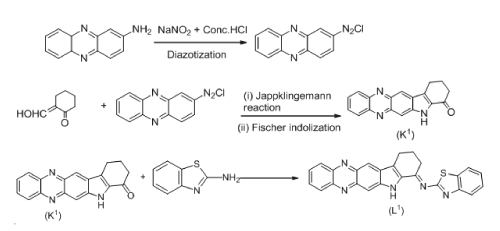
Scheme 1: Synthesis of ligand L1
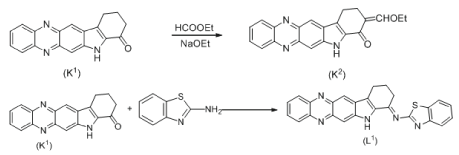
Synthesis of ligand L2
Ligand L2 was synthesized by same procedure methods in scheme 2.
Syntheses of complexes
Syntheses of [Th(L1) 2(NO3)2NO3].6H2O and [Th(L2) 2(NO3)2NO3].6H2O
Synthesis of [Th(L1) 2(NO3)2NO3].6H2O
A methanolic solution of Th(NO3)4.6H2O salt 0.058 g (0.0001 mol) were poured to the methanolic solution of ligand (L1) 0.071 g (0.0002 mol) in 1:2 metal ligand stoichiometry ratio. The reaction mixtures were subjected to energy source of microwave for 5–6 min. The product was recrystallized with mixture of ethanol and ether, and finally dried by rotavapour. The progress of the reaction and purity of the product was monitored by TLC using silica gel [Figure 1].
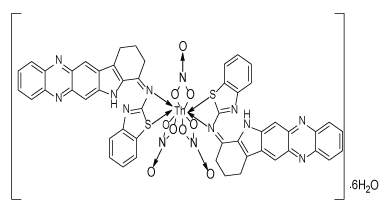
Synthesis of [Th(L1) 2(NO3)2NO3].6H2O
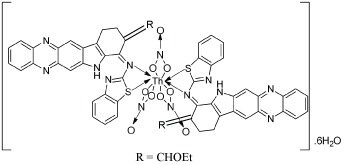
The calculated amount of Th(NO3)4.6H2O 0.058 g (0.0001 mol) and 0.091 g (0.0002 mol) ligand (L2) in 1:2 molar ratio dissolved in methanol stirred for 6 min under microwave irradiation at pH 7–8. The precipitate was filtered and washed with methanol. The product was recrystallized with mixture of ethanol and ether, and finally dried by rotavapour. The progress of the reaction and purity of the product was monitored by TLC using silica gel [Figure 2].
Antimicrobial activity
Antimicrobial activities of the ligands and its complexes were screened by the determination of minimum inhibitory concentration (MIC, μg/ml) using a micro-broth dilution method [17]. A stock solution of each antimicrobial compound in dimethylsulfoxide (DMSO) was prepared according to national committee for clinical laboratory standards guidelines [18,19]. The MIC was determined in standard sterile 96 well flat bottom microtitre plates, and the layout was designed so that each row covered the final antimicrobial dilution of 0.5-500μg/ml with one control well. Using sterilized micropipette 40 μl of the selected antimicrobial with the correct concentration was added to each well, and another well was loaded with the same volume of control (DMSO solvent). Then150 μl of Mueller Hinton media was added to all wells, followed by 10 μl of the respective bacterial suspension to give final concentration of 5×107cfu/ml in each well. The plates were sealed and incubated at 37â°C under atmospheric conditions. After 24 h incubation the microtitre plates were analysed using the ELIZA reader, and the lowest concentration with optical density less than control was defined as the MIC.
Results and Discussion
Elemental analyses, Magnetic properties Molar conductance
Elemental analyses of Th(IV) complexes of ligands are showed 1:1 electrolytic in natures of the complexes [20]. Magnetic moment recorded for Th(IV) complex is due to the presence of one unpaired electron [21]. The calculated and experimental values of CHNSO for ligands and their complexes are agreed with the proposed formulae. Table 1.
Table 1: Physical and analytical data of ligands and their corresponding complexes.
| Compound | Elemental analysis | M.P.(°C) | M.W.found (cal.) | Yield (%) | Time (min.) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Th | |||||
| L1 C21H15N5S |
71.70 (70.20) |
4.86 (4.84) |
- | 16.08 (16.2) |
7.36 (7.41) |
– | 174 | 435.15 (435.44) |
80 | 4–5 |
| L2 C24H19N5OS |
70.85 (70.69) |
5.13 (5.21) |
3.25 (3.48) |
14.25 (14.5) |
6.52 (6.85) |
– | 191 | 491.18 (490.41) |
74 | 5-6 |
| [Th(L1)2(NO3)3]NO3.6H2O | 29.50 (29.48) |
3.32 (3.46) |
13.88 (13.69) |
4.47 (4.44) |
22.60 (22.61) |
16.28 (17.37) |
275 | 1404 (1404.48) |
90 | 5-6 |
| [Th(L2)2(NO3)3]NO3.6H2O | 28.06 (28.11) |
3.55 (3.52) |
14.63 (13.19) |
4.46 (4.60) |
22.23 (22.25) |
17.12 (15.88) |
298 | 1539.29 (1538.1) |
80 | 6 |
Spectral Characterization
FTIR analysis
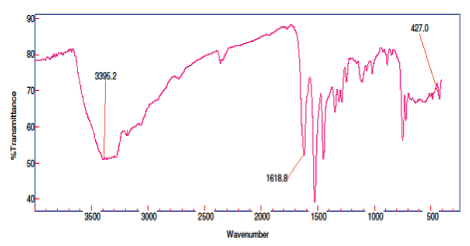
The FTIR spectra of the free ligands were compared with the spectra of their metal complexes. The significant broad band was obtained at 1623–1618 cm–1 [22] corresponding to imine group stretching, which shifted toward lower frequency in metal complexes than the free ligands. The broad absorption band in the region 3395–3288 cm–1 indicates the presence of water molecule in the complexes and results were further confirmed by TGA studies [23]. These bands are conformed the participation of the N and S in the thorium metal complexation. These peaks are expected to be changeduponchelation [24,25]. 427cm–1 corresponding to S group taking the participation to the thorium metal complex [Figure 3,4].
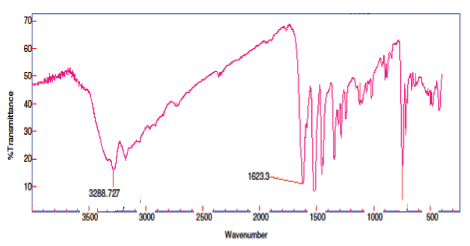
Figure 3: FTIR spectra of ligand (L1).
Electronic spectra
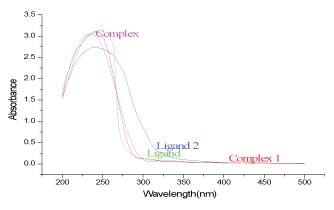
In the electronic spectra, the most characteristic band for the Schiff bases is assigned to π−π* transitionsof the C=N group, which was recorded in the region 252-254 nm. The spectra of complexes indicate slightly shifted 253-256 that thorium has no significant absorption in the visible region due to absence of f−f transition [26]. Figure 5.
NMR analysis
Ananalysis of novel exposed that the NMR spectroscopy has been proved valuable in establishing the character and structure of severalligand as well as its complexes in solutions.
1H NMR spectrum of ligand (L1) (400 MHz, DMSO–d6): δ = 8.85 ppm (s, 1H, -NH, indole), δ = 7.69–7.46 ppm (m, 6H, Ar−H), δ = 7.44–7.03 ppm (m, 2H, thiazole), δ 3.32= ppm (s, 2H, methylene), δ = 2.49 ppm (s, 2H, methylene), δ = 5.03−5.00 ppm (s, 1H, ethylene), δ = 1.01 ppm (t, 3H, methyl). 1H NMR spectrum of metal complex (400 MHz, DMSO‒d6): δ = 8.85 ppm (s, 2H, −NH indole), δ = 7.67–7.61 ppm (m, 12H, Ar−H), δ = 7.43–7.31ppm (s, 4H, thiazole), δ = 3.34‒3.33 ppm (s, 4H, methyl), δ = 5.02‒4.99 ppm (s, 2H, ethylene), δ = 2.49–2.06 ppm (s, 4H, methylene), δ = 1.11 ppm (t, 6H, methyl). Figure 6,7.
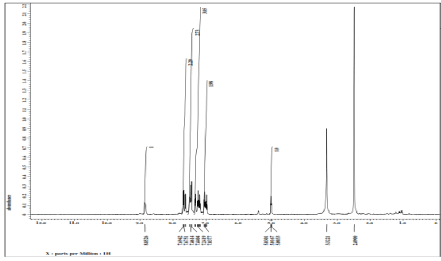
Figure 6: 1HNMR spectrum of ligand (L1)
TGA
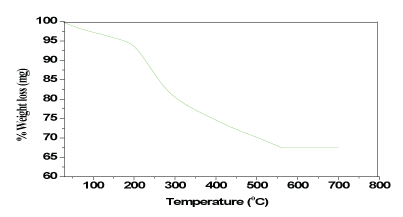
The first step in the temperature range of 110–180â°C with mass loss of 8.51% (calcd: 8.54%) is in accordance with the loss of lattice water molecules. The second step of decomposition occurred within the range of 180-255â°C with a percentage weight loss of 11.93 (calcd: 11.77%) is due to the loss of three coordinate nitrates atoms. The third step of decomposition occurred within the range of 255-310â°C which is considered the loss of 4.31 (calcd: 4.13%) lattice nitrate atom. The fourth observed in the range of 310–400â°C showed a weight loss of 29.55 (calcd: 29.10%) framework of the ligands. The fifth and last step observed range of 400–55â°C 22.39 (calcd: 22.77%) of stable oxide ThO2. Figure 8.
XRD analysis
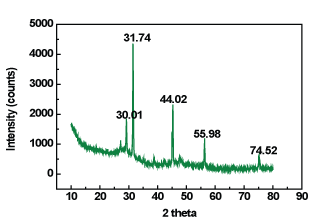
The diffractogram of complex has five reflections with 2θ values from 30.01â° to 74.52â°corresponding to d value 1.2722Å to 3.981Å, respectively. The unit cell of complex yielded values of lattice constants, a = 5.0890Å, b = 5.0890Å, c = 5.0890Å. In concurrence with these cell parameters, and unit cell volume V = 131.79 (Å)3, calculated density 11.69g/cm-3 with these cell parameters, the conditions such as a = b = c and α = β = γ =90â°. the conditions such as α = β = γ = 90â°, required to be cubic crystal system were tested and found to be satisfactory. Figure 9.
Biological activities
Antimicrobial activity
The antibiotics activities of the compounds were assayed using the micro-broth dilution method against gram positive bacteria StaplococcusaureusATCC 6538 and BacillussubtilisATCC 6633, gram negative bacteria Escheriechiacoli ATCC 25922 and yeast like fungi Candida tropicalisATCC 13803, and AspergillusnigerMTCC 282. A comparative study of MIC values of Schiff bases and its complexes are presented in Figure 10 and 11, which are indicated that metal complexes exhibit higher antimicrobial activity than the free Schiff base ligands.
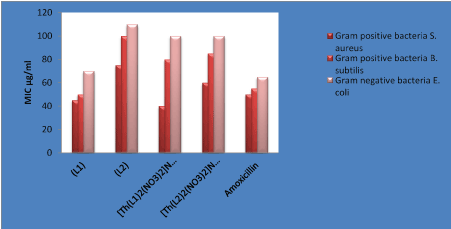
Figure 10: Antibacterial activities against ligands and their metal complexes.
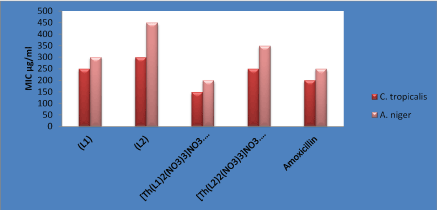
Complexes showed higher activity against both types of bacteria than the free ligand L1 and L2. This enhancement in the activity can be explained on the basis of chelation theory [27]. Chelation reduces the polarity of the Th(IV) ions due to the partial sharing of positive charge with the imine and oxygen donor atoms and possibly the electron delocalization over the whole chelates ring system [28]. Therefore, chelating activities increases the lipophilic nature of the central metal atom, which enhances the penetration of the complexes into the lipid membrane of the microorganism cell wall and thus raising the activity of the complex and restricts the further growth of the organism [29,30].
Conclusion
On the basis of physicochemical and analytical studies discussed, bicapped square antiprismatic geometry for Th(IV) complexes is proposed. Antibacterial and antifungal studies confirmed that the ligands are biologically active and their Th(IV) complexes show enhanced activity against different strains, and same trend was followed for MIC. The interesting properties like porosity behavior may also be investigated in future studies.
Acknowledgements
We are thankful to Professor AdityaShastri, Vice Chancellor of BanasthaliVidyapith for kindly extending the facilities of ‘‘Banasthali Centre for Education and Research in Basic Sciences” sanctioned under CURIE programme of the Department of Science and Technology, New Delhi. The authors are also thankful to Head, Department of Pharmacy Banasthali University for providing antimicrobial facility. We are also thankful vice chancellor Dr. Sachin Gupta from Sanskritiuniversity.
References
- P Guerriero, S Tamburini, PA Vigato (1995) Coord. Chem. Rev. 139: 17.
- Seth P, Giri S, Ghosh A (2015) Tuning of exchange coupling by the Mn-O distance and phenoxido bridging angle: an experimental and theoretical study of the family of Mn(iii) dimers with salen type ligands. Dalton Trans 44: 12863-12870. [crossref]
- MB Lachachi, T Benabdallah, PM Aguiar, MH Youcef, AC Whitwooda, et al. (2015) Dalton Trans. 44: 11919
- PA Vigato, S Tamburini (2004) Coord. Che. Reviews. 248: 1717.
- JJR Frausto da Silva, RJP Williams (1991) The Biological Chemistry of the Elements, Clarendon Press, Oxford, 14: 370.
- Silva F, Campello MP, Gano L, Fernandes C, Santos IC, et al. (2015) Chemical, radiochemical and biological studies of new gallium(III) complexes with hexadentate chelators. Dalton Trans 44: 3342-3355. [crossref]
- DW Jung, DA Yang, J Kim, WS Ann (2010) Chem. Eng. J. 39: 2883.
- AK Nezhad, F Panahi (2011) Green Chem. 13: 2408.
- Silva F, Campello MP, Gano L, Fernandes C, Santos IC, et al. (2015) Chemical, radiochemical and biological studies of new gallium(III) complexes with hexadentate chelators. Dalton Trans 44: 3342-3355. [crossref]
- SC Sivasubramanian, A Kumar (2014) Green Chem. 16: 4266.
- Kostova I, Saso L (2013) Advances in research of Schiff-base metal complexes as potent antioxidants. Curr Med Chem 20: 4609-4632. [crossref]
- Kamatchi TS, Chitrapriya N, Lee H, Fronczek CF, Fronczek FR, et al. (2012) Ruthenium(II)/(III) complexes of 4-hydroxy-pyridine-2,6-dicarboxylic acid with PPh3/AsPh3 as co-ligand: impact of oxidation state and co-ligands on anticancer activity in vitro. Dalton Trans 41: 2066-2077. [crossref]
- R Prabhakaran, P Kalaivani, P Poornima, F Dallemer, G Paramaguru, et al. (2012) Dalton Trans. 41: 9336.
- FH Li, GH Zhao, HX Wu, H Lin, XX Wu, et al. (2006) J. Inorg. Biochem. 100: 36.
- N Mishra, K Poonia, SK Soni, D Kumar, Polyhedron, doi: http://dx.doi.org/10.1016/j.poly.2016.09.045
- Neelima, Poonia K, Siddiqui S, Arshad M, Kumar D (2016) In vitro anticancer activities of Schiff base and its lanthanum complex. Spectrochim Acta A Mol Biomol Spectrosc 155: 146-154. [crossref]
- ter Laak EA, Noordergraaf JH, Verschure MH (1993) Susceptibilities of Mycoplasma bovis, Mycoplasma dispar, and Ureaplasma diversum strains to antimicrobial agents in vitro. Antimicrob Agents Chemother 37: 317-321. [crossref]
- Yamgar RS, Nivid Y, Nalawade S, Mandewale M, Atram RG, et al. (2014) Novel Zinc(II) Complexes of Heterocyclic Ligands as Antimicrobial Agents: Synthesis, Characterisation, and Antimicrobial Studies. Bioinorg Chem Appl 2014: 276598. [crossref]
- Zaltariov MF, Vlad A, Cazacu M, Avadanei M, Vornicu N, et al. (2015) Silicon-containing bis-azomethines: synthesis, structural characterization, evaluation of the photophysical properties and biological activity. Spectrochim Acta A Mol Biomol Spectrosc 138: 38-48. [crossref]
- Yadav M, Mishra N, Sharma N, Chandra S, Kumar D (2014) Microwave assisted synthesis, characterization and biocidal activities of some new chelates of carbazole derived Schiff bases of cadmium and tin metals. Spectrochim Acta A Mol Biomol Spectrosc 132: 733-742. [crossref]
- Chandra S, Sharma AK (2009) Nickel(II) and copper(II) complexes with Schiff base ligand 2,6-diacetylpyridine bis(carbohydrazone): synthesis and IR, mass, (1)H NMR, electronic and EPR spectral studies. Spectrochim Acta A Mol Biomol Spectrosc 72: 851-857. [crossref]
- MS Refatab, SM El–Megharbelac, AMA Adama (2015) Syn. Reacti. Inorg. Met–Org. Nano–Met Chem. 45: 1300.
- I.J. Bellamy (1970) The Infrared Spectra of the Complex Molecules, Second. Wiley Interscience, New York.
- ZM Miodragovic, G Vuckovic, VM Leove, VM Buzash (2000) Synth. React. Inorg. Met-Org. Chem. 30: 57.
- MS Sujamol, CJ Athira, Y Sindhuand, K Mohanan (2012) Spectrochim. Acta 5: 7106.
- GR Reddy, S Balasubramaniana, K Chennakesavulu (2014) J. Mater. Chem. A2: 15598.
- S Agnihotri, K Arora (2010) Chem. Sci. Trans. 7: 1045.
- Agh-Atabay NM, Dulger B, Gucin F (2005) Structural characterization and antimicrobial activity of 1,3-bis(2-benzimidazyl)-2-thiapropane ligand and its Pd(II) and Zn(II) halide complexes. Eur J Med Chem 40: 1096-1102. [crossref]
- N Mishra, K Poonia, SK Soni, D Kumar (2016) Polyhedron 120: 60.
- Obaleye JA, Adediji JF, Adebayo MA (2011) Synthesis and biological activities on metal complexes of 2,5-diamino-1,3,4-thiadiazole derived from semicarbazide hydrochloride. Molecules 16: 5861-5874. [crossref]
