Review on Titanium Oxide Nanoparticles
Mojoyinola Kofoworola Awodele1*, Olusola Akinrinola1*, Samson Akinsanmi Ige1, George Atilade Alagbe1, Omowunmi Folarin1, Ayodeji Oladiran Awodugba1
1 Department of Pure and Applied Physics, Ladoke Akintola University of Technology, Nigeria.
*Corresponding Author:Mojoyinola Kofoworola Awodele, Olusola Akinrinola, Department of Pure and Applied Physics, Ladoke Akintola University of Technology, Nigeria. Tel: +(234)-8067624977; Fax: +(234)-8067624977; E-mail: mkawodele@lautech.edu.ng; oakinrinola40@lautech.edu.ng
Citation: Mojoyinola Kofoworola Awodele, Olusola Akinrinola, Samson Akinsanmi Ige, George Atilade Alagbe, Omowunmi Folarin, et al. (2022) Review on Titanium Oxide Nanoparticles. Nano Technol & Nano Sci J 4: 128.
Received: December 12, 2022; Accepted: December 20, 2022; Published: December 23, 2022.
Copyright: © 2022 Mojoyinola Kofoworola Awodele, Olusola Akinrinola, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The global energy crisis and the demand for clean, and sustainable environments have initiated the search for novel materials and cutting-edge materials. Likewise, the discovery that the properties of materials (i.e., chemical, physical, and biological) at the nanoscale may be quite different from those within a bulk material has engendered several novel materials and increased the application of existing materials across all fields, ranging from the basic environmental application to the most advance technology. Titanium dioxide are naturally occurring transition metal oxide, TiO2 nanomaterials are renowned for their wide range of uses in photovoltaic and photo catalysis processes. In this chapter, the reader should gain a general knowledge of the current research and application of TiO2. The synthesis and fabrication of the material will be discussed, as will new applications and novel research that can be used to increase the efficiency of titanium oxide across every phase of physical and biological research.
Introduction
A material’s physical, chemical, optical, and electrical properties will speak to how suitable the material will be for scientific and environmental applications. For example, one of the most widely used materials is Titanium dioxide, referred to as titania. Titania is a white, opaque, naturally occurring transition metal oxide. TiO2 is a very important nanomaterial that attracts much attention due to its unique properties. It offers tremendous benefits in the transfer of photons, the photochemical synthesis of hydrogen from water, and as a photocatalyst for the destruction ofhazardous compounds. TiO2 is a superior photocatalyst due to its chemical inertness, non-toxicity, and great oxidizing ability of photo generated holes.
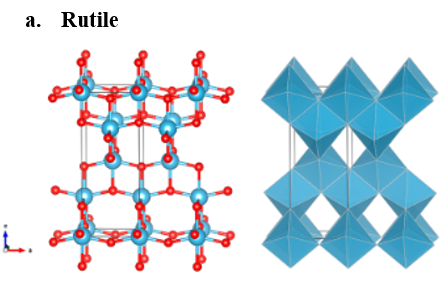
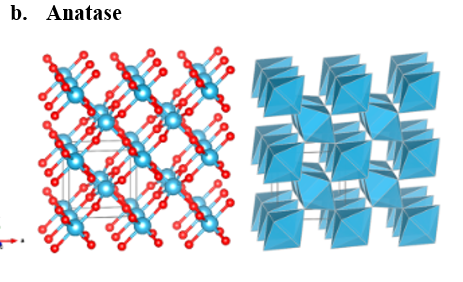
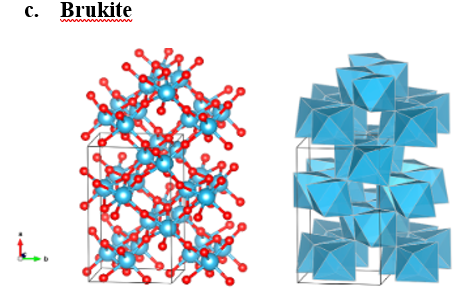
The most significant crystalline forms of TiO2 are rutile, anatase, and brookite, which are shown in fig. 1. These naturally occurring oxide forms are mineable and can be used as a supply of titanium for the industry. Titanium dioxide is both absorbent and odorless. In powder form, its primary application is a common pigment that adds whiteness and opacity [1].
Figure 1: Structural modifications of ???2 [2].
The properties of materials (i.e., chemical, physical, and biological) at the nanoscale are quite different from those within a bulk material [3], TiO2 nanomaterials are renowned for their wide range of uses, which include several environmental (painting, cleaning) and biomedical applications like photocatalytic degradation of pollutants, water purification, biosensing, and drug delivery. These uses span from basic goods like sunscreen to cutting-edge technology like photovoltaic cells. The significance and range of these applications have generated a great deal of attention and significant advancements in the production, characterization, and basic knowledge of TiO2 nanoparticles over the past few decades [4].
Every good thing always has its shortcomings; the impediment to the general application of titanium oxide is the belief that the material has a carcinogenic effect on humans and other microorganisms. However, suppose the material can be inhaled or find a way into the system of an organism. In that case, it causes some chemical changes to the cell, which makes the material useful in biological research, such as cancer treatment [5].
Synthesis of Titanium Dioxide
Titanium metal can be directly oxidized to produce titanium dioxide. However, there are simple alternative processes that can optimize the structure and shape of TiO2 nanoparticles, reduce energy consumption, and utilize low-cost, readily available technology.
Various ways of synthesis affect the size, shape, and Crystallinity of TiO2 nanoparticles. The main methods of obtaining titanium dioxide (with the structure of anatase, rutile, or brookite) in the form of spheres, rods, fibers, and tubes include sol-gel technology, hydrothermal and solvothermal methods, microwave method involving high-frequency electromagnetic waves, template method, electrodeposition, a sonochemical method using ultrasound, chemical, and physical vapor deposition, green methods, etc. In recent research on the synthesis of titania, various techniques have been devised for making mesoporous titania. Comprehensive review of which are documented by [6]. The most frequently used method of synthesizing titanium dioxide in modern scientific research will be examined and summarized below.
Sol-Gel Synthesis Method
A technique for creating solid materials from tiny molecules is the sol-gel process. The technique is used to produce metal oxides, the sol-gel process is a wet chemical procedure that may be carried out at room temperature and is also referred to as chemical solution deposition. The sol-gel process involves several steps in the following chronological order: hydrolysis and polycondensation, gelation, aging, drying, densification, and crystallization.
In 2015, Rasalinga and his teammate carried out an advanced synthesis and pore optimization of a sol-gel synthesized titanate in the same year. The pore modulation was carried out using the hydrothermal method. The team employed a sol-gel synthesis method to produce mesoporous titanium dioxide using 32.5 mL of ethanol and 0.30 mL of conc. HNO3 ethanol and commercially available titanate powder. The resulting gel was then treated with the hydrothermal method overnight at different temperatures of 40°, 90°, 150°, 210°, and 240°C. The characterization result of the five synthesized samples shows that the synthesized samples have the crystallinity morphology of an anatase phase titania, which can be seen in the P-XRD analysis shown in Fig 2 [7].
Figure 2: P-XRD Analysis of different titanium dioxide synthesized at different temperatures.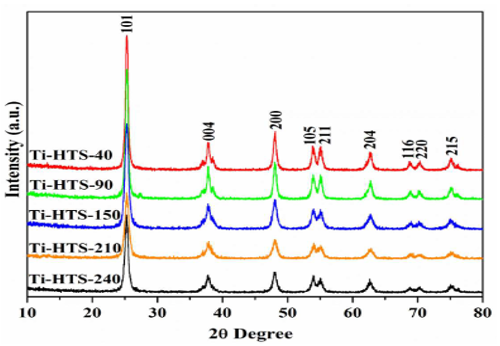
Hydrothermal Synthesis Method
A technique for creating single crystals that relies on the solubility of minerals in hot water under intense pressure is known as hydrothermal synthesis. The crystal growth is carried out in a device called an autoclave, which consists of a steel pressure vessel and is supplied with water and a material. The growth chambers opposite ends are kept at a different temperature. The solute dissolves at the hotter end while it is deposited on a seed crystal at the cooler end, where it grows the desired crystal.
The hydrothermal approach provides advantages over other methods of crystal development, such as the capacity to produce unstable crystal phases near the melting point. The hydrothermal approach also allows for the hydrothermal growth of materials with high vapour pressures close to their melting temperatures.
In 2015, Moazeni and his team worked on synthesizing and evaluating new lithium adsorbents made from titanium dioxide nanotubes, where they employed the hydrothermal method. In the research, hydrogen-based titanate nanotubes were synthesized using the concentrated aqueous NaOH solution and a commercial titanium dioxide powder. For their research, 1.0 g TiO2 (anatase, Merck) was added to an aqueous solution of NaOH, 10 M, 50 ml (pellet, Merck) and stirred with a magnetic stirrer for 1 hour and then placed in an ultrasonic bath (clean-01 elf with input power of 300 VA at rated frequency of 50 Hz) for another 1 hr. The resulting suspension was transferred to a Teflon-lined autoclave in 70 ml volume and heated to 1500C for 48 hr. The result of the characterization of the synthesized titanate nanotube prepared at 1500C for a different reaction time of 24 hours, 48 hours, and 72 hours. It was noted that there are no notable changes in the morphological analysis of the nanotube at higher reaction times. Fig. 3 below illustrates the synthesized titanate nanotube’s SEM analysis at different reaction times [8].
Figure 3: Reported SEM image from 2015 research [8].
Green Synthesis Method
An environmentally acceptable alternative to the chemical method of producing nanomaterials is the green synthesis of TiO2 nanoparticles. Utilizing biological agents like bacteria, fungi, actinomycetes, yeast, and plants. With the assistance of biological agents, as well as ambient temperature and pressure, the rate of reduction process is much faster. The production of TiO2 nanoparticles from plant extracts has significantly advanced the green synthesis method for producing nanoparticles [9].
A green synthesis approach is used to synthesize titanium dioxide nanoparticles (TiO2 NPs) using three different aqueous extracts—pomegranate (Pom), Beta vulgaris (VB), and Seder—Sahar also explore the photodegradation effect of TiO2. Methyl orange organic dye was used as a significant water pollutant in studying the photocatalytic activity of the titanium dioxide nanoparticle [10]. Using various characterization techniques, the morphological structure was found to be tetragonal, consisting of rutile and anatase, with a particle size range of 15.2 to 20.6 nm. The green synthesized TiO2NPs displayed higher photocatalytic activity and efficiency in the photodegradation of methyl orange compared to the chemically synthesized TiO2NPs samples due to the decreased mass percentage of the rutile phase.
Application of Titanium Dioxide in Various Fields
Due to the wide band gap of titanium dioxide, it has found application in various photocatalysis and photovoltaic processes such as degradation of organic waste, abatement of water and air pollutant, artificial photosynthesis, and deactivation of bioparticles. A compelling example of a photocatalysis reaction is “Photosynthesis,” which is the conversion of photon energy (sunlight) into chemical energy (from water and carbon dioxide to glucose and oxygen) and water oxidation [11]. Any reaction that requires photon energy to dissociate or extract an atom is termed Photocatalysis [12,13].
Photocatalytic Application of Titanium Dioxide in Water Treatment
Water is needed for human survival and daily activities; everything we depend on for survival, from planting to washing to cooking, requires water. As a result, the demand for clean, pure, and unpolluted water arises to maintain health. Numerous studies have been conducted to find ways to eliminate impurities that were introduced to natural water by industrial effluents, such as colors from the textile and cosmetics industries and heavy metal ions present in wastewater from the paint, automotive, and electrical industries [14]. Due to its phase stability, effectiveness, low toxicity, and cost-effectiveness, titanium dioxide is well known for its uses as a photocatalytic material for water purification; however, the commercial application of TiO2 is hampered by its wide bandgap and its vulnerability to recombination of the generated electron and its counterpart hole. Researchers have been working non-stop on alleviating these challenges by modulating the pore size of TiO2 and doping titania with other promising materials like graphene and graphene oxide (carbon nanotubes) [15].
In 2018, Korina and his team worked on synthesizing TiO2 coated on magnetic-based polymer fiber core and its application for water treatment [16]. It was discovered that the unique hybrid fiber materials’ thermal characteristics and crystallinity were affected by this structure and the presence of the inorganic components. The produced materials also showed improved tensile strength and magnetic characteristics. Additionally, the materials’ high photocatalytic activity continued even after three uses, thanks to the targeted decorating of TiO2 nanoparticles. Furthermore, the photocatalytic performance of the graphene-oxide was examined, polydopamine, and titanium dioxide nanowire (NPC) membrane with the NWC membrane, which used cellulose acetate membrane as a support layer. The NWC membrane exhibits good potential for water treatment because it has a more stable permeability flux and stable waste removal rate [17].
In 2021, Schnabel and others worked on ablating water pollutants by utilizing TiO2 to degrade non-polar (diesel fuel) and polar substances (methylene blue) in water. According to the study, titanium dioxide-covered foam glass could reduce diesel fuel concentration by 329 mg/L, while coated glass fiber and coated steel grit reduced methylene blue concentration by 96.6 % after 4 hours and 99.1 % after 6 hours, respectively [18].
Photocatalyst Degradation of Air Pollutant
The effects of air pollution on the human body vary depending on the type of pollutant and the length and level of exposure. The increasing urbanization and industrialization have led to the development of harmful environmental conditions such as pollution. Pollutants are made up of tiny particles of chemicals, soil, smoke, dust, or allergens. They can penetrate the lungs and bloodstream and worsen bronchitis, heart attacks and even death. It's even worse for people who have asthma or allergies: these extra pollutants can intensify their symptoms and trigger asthma attacks. Titanium dioxide have employed as a photocatalyst for air purification.
A reactor with UV-irradiated TiO2 catalysts was used to remove polycyclic aromatic hydrocarbons from the air [19]. The naphthalene and 1-methylnaphthalene are the most volatile polycyclic aromatic hydrocarbons, they were selected as model chemicals. Two office spaces were examined; one had a low concentration of polycyclic aromatic hydrocarbons indoor air and measured 73 m3 in size (Room 1), while the other had a high starting concentration of polycyclic aromatic hydrocarbons and was 51 m3 in size (Room 2). Each area had a prototype reactor. Throughout the study, Room 1 stayed closed and was not in use. During business hours, Room 2 was utilized ordinarily. One hour prior to the installation of the reactor prototypes, the initial concentration was measured. After the installation, room air was sampled for polycyclic aromatic hydrocarbons once every week for 4 weeks while the reactors were operating. The reactors were turned off after four weeks, and another sample of the room's air was collected and examined a week later.
Photovoltaic Application of Titanium Dioxide
As technology develops so does the world's demand for energy, titanium dioxide is now used in dye-sensitized solar cells in an effort to increase solar cell efficiency. TiO2 absorbs photons of visible light radiation at energies lower than that of its band gap. The solar spectrum produces electron-hole pairs in the anatase phase at wavelengths less than 387 nm. Titanium dioxide is utilized as a transparent window layer or to decrease the band gap for better solar spectrum absorption. TiO2 is highly resilient against mechanical damage, chemical reactions, and high temperatures. Electrons and holes that are not consumed upon creation in TiO2 will quickly undergo recombination. The use of TiO2 in solar cells has been the subject of various evaluations; among the best in this area are those that reviewed a number of publications recently [20].
Conclusion
The literature, properties, and synthesis of the nanomaterial TiO2 using sol gel, hydrothermal, and green synthesis have all been successfully reviewed. These processes enable the production of titanium dioxide in the form of spheres, rods, fibers, and tubes with an anatase, rutile, or brukite structure. The sol-gel approach modifies the TiO2 nanomaterial's structure and shape, reduces energy expenditures, and makes use of basic and inexpensive technological equipment, although it is a drawn-out procedure. Because it enables the hydrothermal growth of materials with high vapour pressures and the use of three separate aqueous extracts to examine the photodegradation effect of TiO2 nanomaterials, hydrothermal synthesis is more beneficial than other approaches. It is impossible to overstate how useful TiO2 is for commercial purposes because it can eliminate impurities from water and air. Its application in dye sensitized solar cells compared to commercial TiO2 particles, the dye-sensitized solar cells containing TiO2 Nano rods have a greater solar efficiency.
References
1. Haider AJ, Jameel ZN, Al-Hussaini IHM (2019) Review on: Titanium dioxide applications. Energy Procedia 157: 17-29.
2. Zhang H, Banfield JF (2014) Structural characteristics and mechanical and thermodynamic properties of nanocrystalline TiO2. In Chemical Reviews 114: 9613-9644.
3. Behera A, Mohapatra SS, Verma DK (2019) Nanomaterials: Fundamental Principle and Applications. In Nanotechnology and Nanomaterial Applications in Food, Health, and Biomedical Sciences 163-194.
4. Chen X, Selloni A (2014) Introduction: Titanium Dioxide (TiO2) Nanomaterials. Chemical Reviews 114: 9281-9282.
5. Markowska-Szczupak A, Ulfig K, Morawski AW (2011) The application of titanium dioxide for deactivation of bioparticulates:An overview. In Catalysis Today 169: 249-257.
6. Bagheri S, Mohd Hir ZA, Termeh Yousefi A, Bee Abdul Hamid S (2015) Progress on mesoporous titanium dioxide: Synthesis, modification and applications. In Microporous and Mesoporous Materials 218: 206–222.
7. Rasalingam S, Wu C M, Koodali RT (2015) Modulation of pore sizes of titanium dioxide photocatalysts by a facile template free hydrothermal synthesis method: Implications for photocatalytic degradation of rhodamine B. ACS Applied Materials and Interfaces 7: 4368-4380.
8. Moazeni M, Hajipour H, Askari M, Nusheh M (2015) Hydrothermal synthesis and characterization of titanium dioxide nanotubes as novel lithium adsorbents. Materials Research Bulletin 61: 70–75.
9. Aswini R, Murugesan S, Kannan K (2021) Bio-engineered TiO2 nanoparticles using Ledebouria revoluta extract: Larvicidal, histopathological, antibacterial and anticancer activity. International Journal of Environmental Analytical Chemistry 101: 2926–2936.
10. Mousa SA, Shalan AE, Hassan HH, Ebnawaled AA, Khairy SA (2022) Enhanced the photocatalytic degradation of titanium dioxide nanoparticles synthesized by different plant extracts for wastewater treatment. Journal of Molecular Structure 1250:131912.
11. Berardi S, Drouet S, Francàs L, Gimbert-Suriñach C, Guttentag M et al. (2014) Molecular artificial photosynthesis. Chem. Soc. Rev 43: 7501–7519.
12. Yang X, Wang D (2018) Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Applied Energy Materials 1: 6657–6693.
13. AD McNaught, A Wilkinson (2004) Compendium of Chemical Terminology the Gold Book, Second Edition. IUPAC.
14. Mekonnen MM, Hoekstra AY (2016) Sustainability: Four billion people facing severe water scarcity. Science Advances 2.
15. Tayel A, Ramadan AR, el Seoud OA (2018) Titanium dioxide/graphene and titanium dioxide/graphene oxide nanocomposites: Synthesis, characterization and photocatalytic applications for water decontamination. In Catalysts 8 MDPI.
16. Korina E, Stoilova O, Manolova N, Rashkov I (2018) Polymer fibers with magnetic core decorated with titanium dioxide prospective for photocatalytic water treatment. Journal of Environmental Chemical Engineering 6: 2075-2084.
17. Yu Z, Zeng H, Min X, Zhu X (2020) High-performance composite photocatalytic membrane based on titanium dioxide nanowire/graphene oxide for water treatment. Journal of Applied Polymer Science 137: 12.
18. Schnabel T, Jautzus N, Mehling S, Springer C, Londong J (2021) Photocatalytic degradation of hydrocarbons and methylene blue using floatable titanium dioxide catalysts in contaminated water. Journal of Water Reuse and Desalination 11: 224-235.
19. Schnabel T, Dutschke M, Schuetz F, Hauser F, Springer C (2022) Photocatalytic air purification of polycyclic aromatic hydrocarbons: Application of a flow-through reactor, kinetic studies and degradation pathways. Journal of Photochemistry and Photobiology A: Chemistry 430.
20. Omar A, Ali MS, Abd Rahim N (2020) Electron transport properties analysis of titanium dioxide dye-sensitized solar cells (TiO2-DSSCs) based natural dyes using electrochemical impedance spectroscopy concept: A review. Solar Energy 207: 1088-1121.
