Hazardous Acid Neutralization Salt of Lead Batteries in Composites with Galvanic Process Glass Waste and Municipal Water Treatment Sludge Application to Produce Sustainable Ceramics
Vsévolod Mymrin1*, Renata M. Correia1, Fabio L. Cavalin1, Kirill Alekseev1, Rogerio J. Hultmann1, Karina Q. Carvalho1, Fernando H. Passig1, Charles W.I. Haminiuk1, Rodrigo E. Catai1
1Department of Civil Engineering, Federal Technological University of Parana, Str. Deputado Heitor Alencar Furtado, 4900, Campus Curitiba, CEP 81280-340, Ecoville, State of Paraná, Brazil.
*Corresponding Author:Vsévolod Mymrin, Department of Civil Engineering, Federal Technological University of Parana,Brazil, Tel: (55-41) 3232-2568; Fax: +(55-41) 3232-2568; E-mail: seva6219@gmail.com
Citation Vsévolod Mymrin, Renata M. Correia, Fabio L. Cavalin, Kirill Alekseev, Rogerio J. Hultmann, et al. (2022) Hazardous acid neutralization salt of lead batteries in composites with galvanic process glass waste and municipal water treatment sludge application to produce sustainable ceramics. Nano Technol & Nano Sci J 4: 127.
Received: December 12, 2022; Accepted: December 18, 2022; Published: December 22, 2022.
Copyright: Vsévolod Mymrin, et al. (2022). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Hazardous sludge of lead batteries’ acid neutralization salt in composites with glass waste of galvanization process and sludge of municipal water cleaning station with high content of Cl, Ba and Br, was used to produce sustainable ceramics in comparison with traditional natural clay-sand mix. Total content of heavy metals in initial mixtures reached 12.7%. Flexural resistance values of ceramics from traditional clay-sand mix sintered at 900°C was 9.2 MPa and decreased till 8.5 MPa at 1050°C. And vice versa, the developed ceramics only from industrial waste had 8.5 MPa at 900°C and increased till 22.6 MPa at 1050°C. A similar improvement was observed in the study of all physical and mechanical properties of the developed ceramic materials of this research. Investigation of the processes of this compositions’ structure formation with XRD, SEM, EDS, laser micro-mass analysis (LAMMA), and atomic absorption spectroscopy (AAS) methods allowed to determine that in the process of heating, partial melting and thermochemical interaction there is an interaction of the initial components with the synthesis of new minerals - lazurite Na8(Al2SiO4)6(SO4,S)2, magnetite Fe3O4, hematite Fe2O3 and nepheline Na3K(Si0.553Al0.447)8O16 with significant increasing of amorphous materials content. The AAS method showed a very low level of leachate solutions’ contamination compared with the initial raw materials and the requirements of Brazilian environmental standards. Moreover, the use of hazardous industrial and municipal waste, such as acid neutralization salt of lead batteries, glass waste of galvanization process and water treatment sludge of municipal station to produce sustainable ceramic materials can decrease the mining of natural materials and the irreversible destruction of natureby quarries.
Keywords
Lead Batteries’ Acid Inertization Salt; Galvanization Process’ Glass Waste; Municipal Water Treatment Sludge; Industrial Waste;Hazardous Waste’s Thermochemical Interaction; Structure Formation Processes; Environment Protection.
Introduction
This paper presents the development of construction materials from lead batteries’ acid neutralization salts (SAN), galvanization process’ glass waste (GW), municipal water cleaning sludge (WCS) - the byproduct of drinking water production in comparison with traditional clay-sand mixture (CSM). WCS from municipal water plants is one of the most widely distributed types of urban agglomeration wastes all over the world [2]. Vigneswaran and [1] showed that water demand increases due to technological advancement, population growth, and urbanization result in great stress to the natural water cycle. WCS usually includes grit, screenings and sediments [3]. The common practice of WCS treatment is to discharge it directly onto the soil as landfills. But the main part of WCS is characterized by high contents of heavy metals, e.g., Ni, Zn, Cr, Sn, Cu, Pb, Sb, etc. which are extremely hazardous environmental pollutants, studied in detail by [4]. All WCS could and should be used as raw material for production of different environmentally friendly and economically efficient products [4]. [13] proved that the dewatered and dried WCS can be used to remove phosphorus from wastewater treatment plant. [5] used it adding 90 % of natural clay to produce the brick; [6] used WCS ash at a 5% replacement level as a brick-manufacture raw material through a sintering process. WCS can replace 10 – 50 wt% of Portland cement in the production of paving tiles [6] for external use. [11] used WTS for alumina recovery and for production of cement. [7,8] successfully used WCS as additives to cement mortars. [12] evaluate energy potential of WTS generated in the Leningrad Region for Cement production process and for slate processes. [9] used some physical methods (e.g., heating or ultrasonic treatment) for such types of wastes utilization.
The goals of this research are: to develop new environmentally friendly compositions for ceramics production with WCS as the main component (up to 60 wt.%), having acceptable mechanical properties; to study the processes of the new materials’ formation in order to control their mechanical and chemical properties [10]. Red ceramics were chosen as final product, because of their potential to provide effective binding of heavy metals in insoluble state [14-18].
Materials and Methods
Materials
provided by the All-raw materials used in this study were collected in local industrial enterprises of Paraná state, Brazil: WCS was municipal water company SANEPAR, GW – from workshop of glass treatment, SAN – from a recycler of lead-acid batteries; the only natural material used in this study - clay-sand mixture (CSM) - was provided by a local brick factory as its working mixtureof sand and clay. Optimal mixture moisture content was between 10 and 12%. Wet mixtures were pressed at 10 MPa in rectangle form of 60 x 20 x 10 mm and dried at a temperature of 100°C till constant weight. The test samples (TSs) were sintered at 900°, 950°, 1,000°, 1,050° and 1,100°C during 6 hours.
Methods
The raw materials and ceramics were characterized using various methods: XRF to determine the chemical composition; mineralogical composition by XRD; morphological structures by SEM method; isotope compositions by LAMMA-1000 method; solubility and leaching of metals from leachates were studied using AAS method; granulometric composition was determined by laser diffraction particle size distribution analysis; mechanical resistance – by three-point flexural strength method; and the coefficient of water absorption by immersion. Specific gravity was determined by Archimedes' principle using water.
Samples preparation
Samples of ceramics were prepared from the wastes under study by mixing the raw materials in different proportions (Table 1). The CSM - traditionalworking mixture of a ceramic factory - was used in this study as standard (100%) reference, without addition of the industrial wastes (composition 1).
Table 1: Compositions of the test samples (TSs) from the raw materials.
|
N° |
Components, wt. % |
|||
|
WCS |
GW |
SAN |
CSM |
|
|
1 |
0 |
0 |
0 |
100 |
|
2 |
50 |
15 |
15 |
20 |
|
3 |
30 |
25 |
15 |
30 |
|
4 |
35 |
20 |
15 |
30 |
|
5 |
45 |
15 |
15 |
25 |
|
6 |
35 |
15 |
15 |
35 |
|
7 |
45 |
20 |
20 |
15 |
|
8 |
50 |
25 |
25 |
0 |
The content of the components in the mixtures varies within the following ranges: WCS – between 30 and 50wt%, GW – 15-25, SAN – 15-25%.
Calculations
The flexural rupture strength of the TSs was measured following standard NBR 15,270 3/05 (2005) and using the following equation:
RF = 3PL/bh2 Eq. 1
where RF is the flexural rupture strength (MPa), P is the maximum load supported by the specimen (kgf), L is the distance between the supports (mm), b is the width of the TS (mm), and h is the height of the TS (mm). Water absorption (WA) was measured according to the Brazilian standard NBR15270 3/05, (2005), which uses the following equation:
WA = [(MSAT –MD)/MD] x 100 Eq. 2
where MSAT is the mass of the water-saturated specimen after 24 h of water immersion, and MD is the mass of the dry specimen after sintering. Apparent specific density was calculated using the equation
DA = Ps/(Pu – Pi) Eq. 3
where DA = apparent specific density (g/cm³); Ps = mass of specimen sintered and dry (g); Pu = mass of specimen sintered and wet (g); and Pi = mass of the wet specimen immersed in water (g).
Results and Discussion
This experimental research was performed in three steps: physicochemical characterization of the raw materials (WCS, GW, SAN and CSM); preparation and study of the TSs containing different proportions of the wastes; and characterization and evaluation of mechanical, chemical and mineralogical properties after sintering at different temperatures.
Raw materials and preparation of test samples (TSs)
The WCS displays (Table 2) the highest Al2O3 content (24.46%), Fe2O3 (13.00%) followed.
Table 2: Chemical composition of the raw materials used (wt. %), by XRF method.
|
Oxides |
Oxides contents, wt. % |
||||
|
WCS |
GW |
SAN |
CSM |
||
|
SiO2 |
17.01 | 17.01 | 0.34 | 51.98 | |
|
Al2O3 |
24.46 | 0.85 |
- |
20.40 | |
|
TiO2 |
0.40 |
- |
- |
1.02 | |
|
Fe2O3 |
13.00 | 2.24 | 0.36 | 12.93 | |
|
MnO |
3.20 |
- |
- |
- |
|
|
MgO |
0.15 | 2.50 |
- |
1.35 | |
|
CaO |
0.30 | 8.20 |
- |
- |
|
|
Na2O |
< 0.02 |
8.75 | 39.72 |
- |
|
|
K2O |
0.18 | 0.44 | 1.16 | 4.13 | |
|
P2O5 |
0.44 |
- |
0.40 | 0.19 | |
|
SO3 |
0.61 | 0.26 | 25.38 |
- |
|
| C.L.* |
|
0.34 | 44.64 | 8.0 | |
|
Σ |
99.55 | 99.62 | 99.43 | 100.00 | |
Where: C.L.* - Calcination Loss. by CSM (20.40 and 12.93). The GW shows the highest value of SiO2 (76.04%), followed by CSM with 51.98% of SiO2, while the SAN contains the highest values of Na2O (39.72%) and SO3 (25.38%), although the GW also has a high Na2O content (8.75%). The CSM used in this study is a very common mixture of clay with sand that has high iron oxide content, and it is a conventional raw material in brick factories worldwide. SAN has the highest value of calcination loss (C.L. = 44.64%) because of the large content of SO3= 19.38% - the product of sulfuric acid neutralization. WCS also showed a high level of C.L. (39.77%) due to high content of humus and fertilizers, as well as iron or alumina sulfates. Moreover, all industrial wastes under study include heavy metals and poisonous organic impurities. WCS has 0.32% of Cl, 0.12% of Ba and 0.03% of Br; GW has 0,11% of Pb and 0.27% of Cr; SAN has Sn - 0,25; Sb - 0,38%; Zn – 0.58%; Cu – 1.18%; As-0,47%; Pb - 1,25%; Ba - 0,18%; Cl - 8,24%and Br - 0,04% with total content of extremely hazardous elements 12.57%. Therefore, they are classified as Class I (dangerous) and Class II-A (no inert) materials, according to Brazilian standards NBR 10.004 (2004. The values of metal leaching (Table 3) from all the wastes used as raw materials in this study exceed significantly the allowable levels in national sanitary standards, in particular for as (from WCS and SAN), for Ba (from WCS, GW and CSM), for Pb and Cr from all components.
Table 3: Results of leaching and solubility tests of the raw materials under study.
|
Elements |
Leachate from raw materials, mg/L |
NBR 10,004 |
|||
|
WCS |
SAN |
GW |
CSM |
||
|
As |
17.69 |
1.43 | 0.86 | 0.00 | 1.0 |
|
Ba |
93.37 | 54.87 | 92.72 |
8.16 |
70.0 |
|
Pb |
29.82 | 12.41 | 1.024 |
0. 03 |
1.0 |
|
Cr |
179 |
981 |
14.61 |
0.21 |
5.0 |
|
Elements |
Solubility of metals in raw materials, mg/L |
|
|||
|
WCS |
SAN |
GW |
CSM |
NBR 10,004 |
|
|
Al |
23.17 | 19.34 | 0.45 | 2.09 | 0.2 |
|
Ba |
8.15 | 4.19 | 4.19 |
10.15 |
0.7 |
|
Pb |
27.34 | 18.47 | 1.205 | 4.25 | 0.01 |
|
Cu |
43.15 | 23.78 | 463.25 |
0.15 |
2.0 |
|
Cr |
243.67 | 15.18 | 42.57 |
0.42 |
0.05 |
|
Fe |
3.583 | 1.485 | 82.13 |
15.73 |
0.3 |
|
Mn |
314.22 | 217.97 | 37.58 | 2.19 | 0.1 |
|
Zn |
387.28 | 153.17 | 46.84 |
2.09 |
5.0 |
A large deviation above leaching rate limits established by sanitary standards is found with all raw materials under study (Table 3), particularly from WCS for Pb in 2,734 times, for Cr in 4,873, for Mn in 31,422 times, etc.; from SAN for Mn in 2,180 times, for Pb in 1,847 times, etc. Interpretation of X-ray diffraction patterns of the initial components (Fig.1) shows the presence of the following minerals: WCS - illite KAl2(Si3Al) ·O10(OH)2, kaolin Al2Si2O5(OH)4 and quartz SiO2; SAN - thenardite Na2 SO4 and halite NaCl; GW – quartz and cristobalite SiO2; CSM - illite KAl2(Si3Al) O10(OH)2,magnetite Fe3O4 and quartz SiO2.
Figure 1: X-ray diffractogram patterns of the raw materials: A – WCS, B – SAN, C – GW, D – CSM, E - of the ceramic 8 after sintering at 1,050°C.
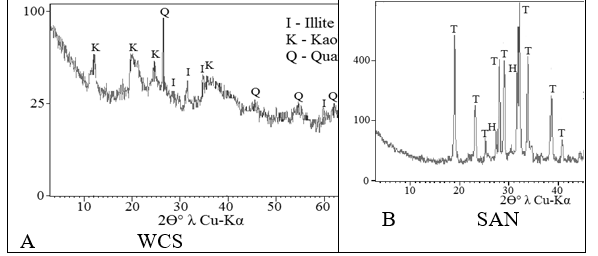
All the initial components used also have different amounts of amorphous material. Its maximum content is observed on the diffractogram patterns of WCS and GW (Fig.1 – A and C) with very weak traces of crystal structures. Very high halo between 11° and 39° of 2θ°λCu-Kα also confirms the presence of glass structures in GW. The smallest amount of amorphous material is observed in the diffractogram pattern of SAN, with a rather high level of mineral crystallization (Fig.1-B). Large amounts of amorphous substances were also observed in CSM as a result of geological processes in the lithosphere.
Figure 2: SEM micro images of the raw materials used: A – WCS, B – SAN, C – GW, D – CSM.
The raw materials under study (Fig.2) exhibit different particle sizes and shapes, along with pores of widely varying shapes and dimensions. Each of the three raw materials under study shows particles not chemically linked, but having only mechanical contacts with each other. GW particles (Fig. 2-C) are distinguished among other raw materials as a mixture of perfectly regular spheres with diameter 12 - 55 microns and their fragments; the mixture includes also a large number of sharp-edged sand particles of various sizes from 1 to 80 microns. SAN particles precipitated (Fig. 2-B) during neutralization of the sulfuric acid lead batteries with alkali have smoothed shapes near 3-5 µm. WCS and CSM do not show a pattern in particle sizes and shapes.
Mechanical properties of the ceramics developed
The values of mechanical properties and standard deviations were obtained as an average of measurements taken in 10 samples. Therefore, the total amount of replication involved in the testing reaches 500 samples. It was shown (Fig. 3 and Table 3) that the resistance of all compositions increased with the decrease in sum of GW and SAN content and increasing with sintering temperature up to 1,050°C.
Figure 3: Flexural strength of the ceramics sintered at different temperatures. 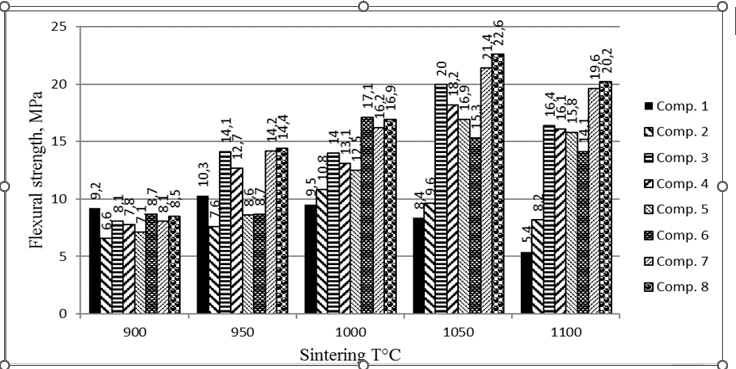
TSs of composition 2 with GW+SAN=30% have the lowest resistance values at all sintering temperatures. They are followed up to 1,000°C by the TSs with the same sum GW+SAN=30%, but with increasing CSM and decreasing WCS content to 5% (composition 5). The highest values of flexural strength are seen with TSs of composition 8, having the highest sum of GW+SAN=50% and WCS (50%) without CSM (Table 3), reaching a maximum resistance of 22.6 MPa (Fig.3). That means that WCS can completely substitute the traditional mixture of natural clay and sand for red ceramics, if sintering is done at 950°C and higher. The resistance of composition 8 is followed by composition 7 with GW+SAN=40%, 45% of WCS and 15% of CSM at almost all temperatures.
The comparison between the flexural strength values of traditional reference ceramics (composition 1) and ceramics developed from mixtures of industrial wastes used here demonstrated great advantage of the latter at temperatures above 900°C with increasing of refractory properties (melting points) of ceramics from 950° till 1,000° – 1,050°C. Thus, the resistance values of reference ceramics and of ceramics produced with the industrial waste are significantly higher than that specified by Brazilian standard NBR 15,270-3.
The standard deviations of the TSs do not exceed the level of 2.5%, therefore they are not shown here. The values of shrinkage coefficient of all ceramics get higher when increasing the sintering temperatures, but in different scales: the reference ceramics (composition 1) have these changes between 1.45 – 2.98%, while ceramics with inclusion of industrial wastes have these changes between 5.3 – 10.7%. This fact can be explained by the presence of salts in SAN and organic substances in WCS. Their release during firing and contraction of the TSs during cooling explains this difference between shrinkage values. Therefore, the biggest content of WCS + SAN = 75% in ceramic 8 leads to the highest shrinkage coefficient (10.7%) at the highest sintering temperature of 1,100°. It is followed (10.3%) by composition 2 with WCS + SAN = 65% and SC=10.3% at 1,100°C. Ceramic 7 has the same WCS + SAN (65%), but it has 5% less content of CSM with own organic content, therefore its SC is equal to 9.9%.
The values of water absorption WA (Fig.4) of reference ceramics with increasing firing temperature gradually decrease from 9.8 to 8.7%. The maximum value of WA = 9.3% was observed at 900°C in samples of composition 8 with a sum of WCS + SAN = 75%, followed by 8.4% of composition 7 with WCS + SAN = 65%. The lowest value of WA at all temperatures was seen in TSs of ceramic 3 with the lowest WCS + SAN of 45%. This means that increasing the sum of WCS + SAN, not only increases the flexural resistance and shrinkage of TSs, but also water absorption. Thus, all compositions met the requirements of the Brazilian standard NBR 15270-3, which specifies water absorption values between 8 and 22%.
Figure 4: Water absorption of ceramics after sintering at different temperatures.
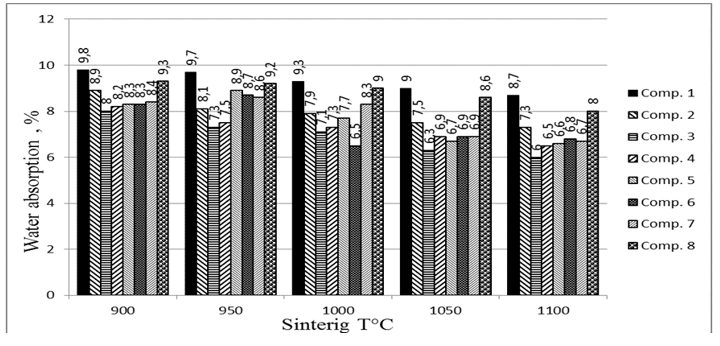
Figure 5: Changes in ceramics bulk density after sintering at different temperatures.
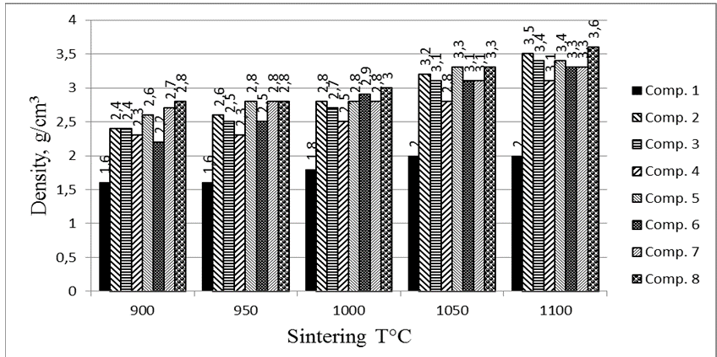
the density value (Fig. 5) was found to depend not only on the firing temperature, but is also directly proportional to the amount of WCS and SAN additives introduced in the samples, in much the same way as mechanical properties. Therefore, TSs of composition 8 with WRS + SAN=75% achieved the maximum bulk density values at all sintering temperatures, particularly 2.8 g/cm3 at 900°C and 3.6 g/cm3 at 1,100°C. The high bulk density of other ceramics at all temperatures are comparable to the densest TSs of composition 8.
Physicochemical processes of ceramic’s structure formation
The ceramic of composition 8 (consisting only of industrial wastes) has the best mechanical properties at all sintering temperatures, so it was chosen to study the processes of structure formation by the methods of XRD, SEM, EDS, LAMMA and AAS. Interpretation of X-ray diffraction patterns of composition 8 TSs after sintering at 1,100°C
during one hour (Fig.1 - E) shows the presence of the following minerals: mullite 3Al2O3·2SiO2, quartz SiO2, nepheline (Na,K)AlSiO4, corundum ?l2?3 and lazurite Na8(Al2SiO4)6(SO4,S)2. The absence of CSM with its magnetite among the raw materials of the initial mixture of composition 8 leads to the absence of hematite in the final ceramic.
Figure 6: SEM micro images of the TSs of composition 8 after sintering at 1,050°C.
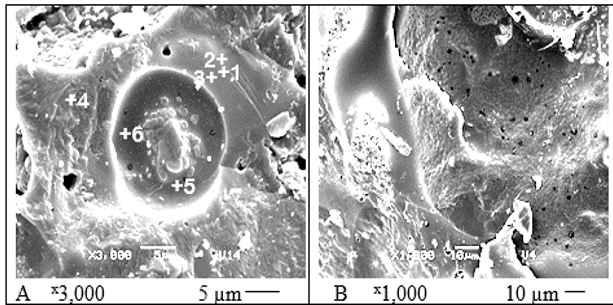
MEV images of ceramic 8 microstructures (Fig. 6) after firing at 1,050°C for one hour show completely molten surfaces of initially separated particles (Fig. 2). All of them were transformed into continuous glassy material with closed and open pores of different sizes and forms. The surface of composition 8 (Fig.6) seems like frozen waves of early melted materials. Some of the pores also appeared due to chemical interaction of components at high temperatures with formation and release of gases. Others have semi-spherical or oval shape typical of totally melted materials. The heterogeneity of chemical composition of ceramic new formations was also studied by laser micro-mass analysis (LAMMA), which provides isotopic combinations at the focus point. All three analyzed points of the TS of composition 8 after sintering at 1,100°C show (Fig. 7) quite a different set of isotopes. The intensity of the peaks of each of the isotopes on three graphs are significantly different from each other, indicating their large quantitative difference. Thus, the LAMMA method results reliably confirm the conclusion obtained by EDC method, that of predominant heterogeneity (amorphous nature) of the chemical composition of new ceramic formations.The color and gloss level of the materials vary from light brown to dark brown, depending on the components’ percentage, therefore it is rather aesthetically pleasing. This color transformation occurred due to the transition of small quantity (below 5% - the level of sensibility of XRD method) of hematite Fe2O3 during the ceramics sintering to more dark magnetite Fe3O4.
Figure7: Isotope contents in ceramics of composition 8 after sintering at 1,050°C.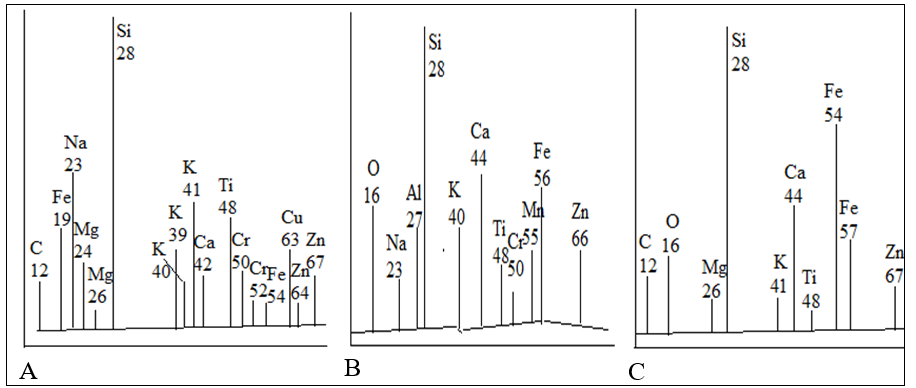
Leaching and solubility tests.
The values of leaching and solubility tests were determined by AAS method. The comparison of experimental values of heavy metals’ leaching and solubility (Table 4) to demands of the Brazilian standards shows a big (up to 700 times for leaching of Ba) advantage in the results.
Table 4: Results of leaching and solubility tests of composition 8 after sintering at 1,050°C.
|
Elements |
Leaching, mg/L |
Solubility, mg/L |
|||
|
NBR* |
Elements |
NBR* |
|||
|
As |
0.35 | 1.0 |
As |
< 0.001 |
0.01 |
|
Ba |
< 0.1 |
70.0 |
Ba |
< 0.1 |
0.7 |
|
Cd |
< 0.005 |
0.5 |
Cd |
< 0.005 |
0.005 |
|
Pb |
< 0.01 |
1.0 |
Pb |
< 0.01 |
0.01 |
|
Cr |
0.61 | 5.0 |
Cr |
< 0.005 |
0.05 |
|
Hg |
< 0.0002 |
0.1 |
Hg |
< 0.0002 |
0.001 |
|
Se |
< 0.001 |
1.0 |
Se |
< 0.001 |
0.01 |
|
Note: NBR * - Brazilian standard NBR 10,004 |
Al |
< 0.1 |
0.2 | ||
|
Cu |
< 0.01 |
2.0 | |||
|
Fe |
0.3 | ||||
|
Mn |
< 0.01 |
0.1 | |||
|
Zn |
5.0 | ||||
obtained. This number can reach thousands of times if compared to leaching and solubility from the raw materials (Table 2). This fact confirms the strong chemical binding of metals into a chemically stable state during the thermochemical processes of ceramic sintering. It is possible to affirm, based on the results of leaching and solubility tests (Tables 4) and considerable world technical literature, that the materials developed in this work, at the end of their service life, can be successfully recycled as valuable components of new materials.
Conclusions
1. It is experimentally proved that sediments of municipal water treatment plant can completely substitute traditional mixtures of natural clay and sand for red ceramics sintering at 950°C and higher. The values of flexural resistance strength of ceramics from traditional mixtures at 1,100°C is 8.4 MPa and of ceramics from only industrial wastes reaches 22.6 MPa.
Acknowledgments
The authors are indebted to the Laboratory of Minerals and Rocks (LAMIR) and to the Laboratory of Environment Technology, both of UFPR, Curitiba, Brazil, due the strong technical assistance in performance of analyzes.
References
- Vigneswaran, S Sundaravadivel, M (2004) Recycle and reuse of domestic wastewater waste water recycle, reuse and reclamation, Encyclopedia of Life Support Systems (EOLSS), UNESCO.
- Chu CP, Lee DJ, Chang CY (2005) Energy demand in sediments dewatering, Water Research, 39: 1858-1868.
- Twort, A Rathayaka, D Brandt, M J (2005) Water Supply, Fifth Editor. IWA Publishing ISBN, 340 72018 2.
- Richter CA (2004) Treatment of sludge from wastewater treatment plant, Edgard Blücher Ltda, São Paulo.
- Anyakora, NV (2013) Characterization and performance evaluation of water works sludge as bricks material, J. Eng. Applied Sci 3: 3.
- Chihpin, H Ruhsing, PJ Sun, K D Liaw CT (2013) Reuse of water treatment plant sludge and dam sediment in brick-making. J. Eng. Appl. Sci 3: 3.
- John, VM, Angulo, CS, Zordan SE (2001)Desenvolvimento Sustentável e a Reciclagem de Resíduos na Construção Civil. In: Seminário desenvolvimento sustentável e a reciclagem na construção civil – materiais reciclados e suas aplicações, São Paulo.
- Abdul, G, Liew, AI, Calvin, HKW, Abdul, AS (2004) Incorporation of sewage sludge in clay brick and its characterization. J.Waste Manag. Res 22: 226–233.
- Degremont O. Water Treatment Handbook, France, 2007. ISBN 978-2-74030-0970-0, 2007.
- Mymrin V, Borgo, C, Ponte, HA (2006) Galvanic processes waste as principal components of red ceramics production, J. Cerâmica Industrial 11: 43-46.
- Kyncl M (2008) Opportunities for water treatment sludge re-use, J. GeoScience Engineering 1: 11-22.
- Deviatkin, I (2013) Wastewater Treatment and Deinking Sludge Utilization Possibilities for Energy and Material Recovery in the Leningrad Region, Lappeenranta University of Technology, Faculty of Technology, 2013, Master’s thesis, 111 pages.
- De-Bashan, L Bashan, Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer. J. Wat. Res., 38: 4227-4246.
- Alqam, M, Jamrah, A, Daghlas, H (2011) Utilization of Cement Incorporated with Water Treatment Sludge. Jordan J. Civil Eng 5: 268 – 277.
- NBR (2004) Solid wastes: classification, Rio de Janeiro.
- NBR, (2005) Flexural Rupture Strength Measurement and Water Adsorption Measurements of Ceramic Bricks, Rio de Janeiro: 15270-3.
- Szyczewski, P Siepak, J Niedzielsk, PI Sobczy?ski, T (2009) Research on Heavy Metals in Poland. Polish, J. Environ.Stud 18: 755-768.
- Twort, A Rathayaka, D Brandt M (2000) Water Supply, fifth ed. Arnold Publishing, London, ISBN 0-340-72018-2
