Usefulness of Fluorescence-Guided-Surgery in Achieving Gross Total Resection of Malignant Glioma: Evaluation Using Mr Volumetric Study
Marymol Koshy*1,WP Ng2,BS Liew2,Azmin Kass R2,Mohammad Hanafiah1
1Neurology and Clinical Neurophysiology, Casa di CuraPolispecialisticaSant’Elena, Viale Marconi 160, 09045 QuartuS’Elena, Cagliari, Italy
*Corresponding Author:Marymol Koshy, Associate Professor of Radiology and Senior Consultant Radiologist,Medical Imaging Unit, Faculty of Medicine, Universiti Teknologi MARA, Sungai Buloh Campus, Selangor, Malaysia, ; TEL:603-6126 5000 ; FAX:603-6126 5000;E-mail:marymolkoshy@yahoo.com.sg
Citation:Marymol Koshy, WP Ng, BS Liew, Azmin Kass R, Mohammad Hanafiah, et al. (2016) Usefulness of Fluorescence-Guided-Surgery in Achieving Gross Total Resection of Malignant Glioma: Evaluation Using Mr Volumetric Study. Archiv Neurol Neurosurgery, 1:105.
Copyright:© 2016 Marymol Koshy, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received date:December , 2016; Accepted date:December , 2016; Published date:December , 2016
Abstract
Introduction: Malignant gliomas are highly infiltrative and aggressive primary brain tumors. Achieving gross total resection (GTR) using conventional white light microsurgical technique is a challenge. Five-aminolevulinic acid (5-ALA) can be used as an adjunct for the surgery of adult malignant glioma and improves the rate of gross total resection and patient survival. The use of this method in clinical practice is relatively new in Malaysia. We evaluate the extent of malignant glioma resection under fluorescence-guided resection (FGR) using volumetric MR neuroimaging.
Methodology: A prospective pilot study was carried out in 5 newly diagnosed malignant glioma patients that underwent FGR using 5-ALA. All cases were subjected to both pre-and postoperative MR that was performed 72 hours prior to and post-surgery. The volumetric assessment was performed using special software program. The Extent of Resection (EOR) was then classified into three categories: Gross total resection (GTR, >90% tumor removal), Subtotal resection (STR, resection of 10-90% of tumor) and Partial resection, <10% tumor removal)
Results: Five patients (mean age 54 years, range 45–60 years), 3 males and 2 females were recruited and analysed. These patients harbored Grade IV glioblastoma. The location of the tumor was predominantly in the frontal lobe (n =3, 60%). The median preoperative tumor volume was 35.67cm³ (range 19.4-95.79) and the median postoperative tumor volume was 1.47cm³ (range 0.12-2.37). GTR of >90% was achieved in all 5 patients.
Conclusion: Our experience using Fluorescence-guided surgery enabled a GTR in 100% patients with glioma. We advocate increasing the sample size, which in turn will increase the power of the statistical analysis. The application of 5-ALA has a great potential as a novel standard in neurosurgery in Malaysia to maximize tumor resections for malignant gliomas.
Keywords
5-aminolevulinic acid; glioma; GBM; fluorescence-guided surgery
Introduction
Malignant gliomas and metastasis are the two most common intracranial lesions encountered in the neurosurgical routine [1]. High-grade malignant gliomas are the most common primary brain tumors in adults [2]. Gliomas are tumors, which arise from astrocytes and are highly malignant as the cells reproduce quickly and are nourished by an ample network of blood vessels. As gliomas have finger-like tentacles, they are extremely difficult to remove completely. This becomes a problem especially when they are growing near eloquent areas of brain, which control important functions such as language and coordination. Based on WHO classification, malignant gliomas are comprised of anaplastic astrocytoma (WHO grade III) and glioblastoma (WHO grade IV) [3]. Malignant gliomas may develop at all ages, the peak incidence being in the fifth and sixth decades [4].
Although gliomas rarely metastasizes, they are notoriously invasive tumors and usually spread nearby along the axonal and basal membrane-like structures, where invasive tumor cells have often been identified few centimeters away from the enhancing tumor mass and even on the contralateral side of the brain. The characteristics of glioma cells, which include very high proliferation rate, genetic variability and discernible invasive tendency, leave very few options for therapy. Complete surgical removal of the tumor is key to Improved patient prognosis [5].
Hence the only accepted strategy as far as surgery is concerned is achieving gross debulking of the tumour. The aim of surgery is to remove as much of the tumour as possible while preserving the neighbouring healthy brain tissue. Removal is often complicated by the nature of the tumor especially if the tumor is invasive and doing a complete removal can be difficult. One and two-year survival rates of high grade glioma patients are only 53.7% and 14.6% respectively [6]. A significant survival advantage was associated with resection of 98% or more of the tumor volume [7].
Gross tumor resection (GTR) has been reported to be one of the most important factors affecting the prognosis and survival rate of glioma patients. Due to the infiltrating nature of glioma GTR remains a notorious challenge to neurosurgeons to distinguish between the tumor boundary and the surrounding edematous brain parenchyma. This can result in unintentional removal of healthy tissue or failure to remove malignant tissue [8,9]. In subtotal resection of glioma postoperative magnetic resonance (MR) images have revealed residual contrast enhancement at the resection margins, showing the difficulty of precisely identifying between the areas of infiltration and edematous brain intraoperatively.
In recent years one of the most groundbreaking methods that has come to the forefront in the advancement of local control of glioma surgery is the use of 5-aminolevulinic acid (5-ALA) induced fluorescence to guide surgery. After systemic or topical administration of 5-ALA neoplastic cells synthesize abundant intra -cellular protoporhyrin IX (PpIX). The role of 5-ALA is not only important in resection of gliomas but also for intraoperative visualization of anaplastic regions [5]. During intraoperative tumor resection, brain shift complexes the accuracy of the neuronaviagation system up to several centimeters. 5-ALA fluorescence guided surgery (FgS) of malignant gliomas allows real-time intraoperative assistance for visualization of the tumor. PpIX fluorescence permits differentiating normal tissue from tumor that is independent of neuronavigation and brain shift [10].
Materials and Methods
The objective of this study was to evaluate the extent of malignant glioma resection under fluorescence guided surgery using 5-ALA and performing early pre-and postoperative MR neuroimaging in defining the volumetric extent of resection.
In this prospective study, patients aged above 18 years of age who were able to give consent, with newly diagnosed high-grade supratentorial malignant glioma, and without previous treatment, intended for surgery were considered. Patients with hypersensitivity to porphyrins, porphyria, photosensitivity, exfoliative dermatitis, renal or hepatic impairment or other malignancies were excluded. Patients who were pregnant and unable to undergo MRI due to presence of cochlear implants, surgical metallic clips, cardiac pacemaker or any metallic foreign body were also excluded.
5-ALA (Gliolan) for FgS in malignant glioma is approved for all patients older than 18 years of age. 5-ALA is a natural metabolite in the hemoglobin metabolic pathway found in the body. After oral ingestion it is taken up by glioma cells as it penetrates the blood-brain barrier [11]. It is then metabolized into its fluorescent metabolite, protoporhyrin IX (PpIX). When excited with 405-nm wavelength blue light PpIX emits violet-red fluorescence [12]. Excretion of PpIX is via a hepatic metabolism and normal serum PpIX levels are reached after 24 hours. Patients were protected against direct sun light the following day to prevent sun burns caused by the elevated PpIX in the patient’s skin. Elevated liver enzymes can be found the following days. The flow of surgery is barely disrupted as converting from standard white light to fluorescent mode on the microscope is achieved by the push of a button. Figure 1

Figure 1: A. T1-weighted axial gadolinium-enhanced magnetic resonance image demonstrates an enhancing tumor (arrow) in the left temporal lobe. B. Intraoperatively using conventional white light illumination the infiltrative nature of the malignant glioma tumor (arrow) cells produces indistinct borders between normal and malignant tissues, and the lack of easily identifiable tumor margins confounds attempts at total resection C Under blue light illumination, using oral 5-ALA results in fluorescence of the malignant cells. The malignant cells appears pinkish (arrow) due to 5-ALA induced Protoporphyrin IX, thereby providing an opportunity for more complete tumor resection.
A prospective study was carried out in 5 newly diagnosed malignant glioma patients that underwent fluorescence guided surgery using 5-ALA. Oral administration of 5-ALA at 20 mg/kg body-weight, diluted in 50 mL of water 3h (range 2-4) before induction of anesthesia [13]. Craniotomy was done following general anaesthesia. Surgery was then performed using a modified neurosurgical microscope (Leica), equipped with a fluorescence kit. Surgeons trained in the use of 5-ALA carried out all operations. Each resection was initially performed using a conventional illumination. After the exeresis was judged as complete, the cavity was systematically inspected in the violet- pink light and with the neuronavigation system in order to identify any residual tumor.
All cases were subjected to both pre and postoperative MR that was performed 72 hours prior to and post-surgery. The tumor volume was defined as the volume of the enhancing regions including any rim enhancing necrotic or cystic components depicted on post contrast T1 MPRAGE sequence. Malignant gliomas on MR are depicted as well circumscribed tumors with regional necrosis surrounded by viable invading tissues and marginal contrast uptake [14,15].
All patients were imaged using 1.5T Siemens AERA. Head coil was used in all patients. Prior to the scan patients were explained to about the procedure including possible risk of contrast material administration and written consent was taken in all cases. The volumetric assessment was performed using OsiriX computed software program. After defining the border of the tumour as the region of interest on a slice-to-slice basis (axial plane) manually, this software program allowed for measurement of the total tumour volume. The slice thickness of the T1 MPRAGE images was 1.25mm.
The Extent of Resection (EOR) was calculated using the following formula:
(Preoperative total tumor volume – Postoperative total tumor volume)
Preoperative tumor volume x 100%
EOR was then classified into three categories: Gross total resection (GTR, >90% tumor removal), Subtotal resection (STR, resection of 10-90% of tumor) and Partial resection, <10% tumor removal). GTR is defined as absence of contrast uptake in the area of the GBM following resection, on post-operative T1-weighted MRI obtained within 72 hours’ post-surgery. If there was any doubt about the significance of a contrast-enhancing element, this was considered residual tumour.
Two radiologists experienced in reporting MRI brain independently interpreted the images. In the event of a disagreement a third radiologist independently interpreted the images. Final report was then being made by consensus. All radiologists were blinded from each other’s report. Figure 2
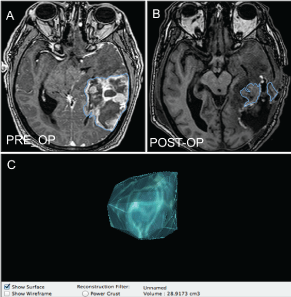
Figure 2: Selected axial T1-MPRAGE Post gadolinium MRI images of a gliobastoma maltiforme pre-operatively (A) and post-operatively (B) together with(C) an example of volumetric reconstruction of selected section of a mass.
Results
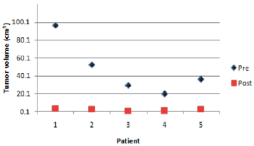
Five patients (mean age) of which 3 males and 2 females were recruited and analysed. The median age was 54 years (range 45–60 years). All the five patients were affectec by newly diagnosed gliomas. Histopathological results revealed that all these patients harbored glioblastoma (WHO grade IV). The location of the tumor was predominantly in the frontal lobe (n =3, 60%). The median preoperative tumor volume was 35.67cm³ (range 19.4-95.79) and the median postoperative tumor volume was 1.47cm³ (range 0.12-2.37). The Gross total resection (GTR) of >90% was achieved in all 5 patients. Table 1 Figure 3
Table 1: Patient Demographic and Clinical characteristics.
| Patient No | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age | 60 | 59 | 51 | 55 | 45 |
| Sex | Male | Male | Female | Female | Male |
| Location | Tempero-parietal | Temporal | Frontal | Frontal | Fronto-parietal |
| Histo-Pathology | Glioblastoma | Glioblastoma | Glioblastoma | Glioblastoma | Glioblastoma |
| Extent of Resection (%) | 97.5 | 96.8 | 99.6 | 98.5 | 95.9 |
Discussion
Maximal possible cytoreduction without incurring neurological deficit in malignant glioma surgery is an independent prognostic factor. Aside from that GTR is an independent prognostic factor for survival in GBM and time to tumor progression (TTP). GTR of 98% and more is considered to have the better prognosis following GBM resection and is best assessed on pre-and post-surgery MRI.
Previous studies have shown that the rate of complete resection using conventional white light microsurgical technique varies between 23-36%. In our study, GTR of >90% was achieved in all 5 patients. Oral administration of 5-ALA increases the extent of resection, as switching to violet-blue light illumination intra-operatively allows the neurosurgeon to better distinguish the glioma margin from normal brain parenchyma. Complete resection results in improved patient survival and greater response to adjuvant therapies. For detection of residual tumour, early postoperative MR can avoid benign enhancement of surgical site caused by blood-brain barrier disruption, surgical manipulation, postoperative inflammation and luxury perfusion. Therefore, it is recommended that early postoperative MR be performed within the first 3 days following surgery, hence minimizing interpretation inaccuracies caused by surgical artifacts, as decision of further therapy is highly dependent on accurate post-operative assessment. A limitation of FgS is that high-grade glioma is often a heterogenous tumour consisting of areas of highly proliferative region, low-grade differentiation and necrosis. The areas of low-grade differentiation and necrosis will not show PpIX fluorescence hence failing to meet the target of gross total removal in a reasonable number of cases.
Conclusion
The findings in this study demonstrates the enormous potential of 5-ALA for visualization of tumor tissue intraoperatively. Fluorescence-guided surgery enabled a GTR in 100% patients. Performing early postoperative MR imaging yielded reliable and accurate information in detecting residual tumor. However due to small sample, the study was underpowered, and thus, did not reach statistical significance. We advocate to increase the sample size, which in turn will increase the power of the statistical analysis. The application of 5-ALA has a great potential as a novel standard in neurosurgery in Malaysia to maximize tumor resections for malignant gliomas.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coburger J, Engelke J, Scheuerle A, Thal DR, Hlavac M, et al. (2014) Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus 36: E3. [crossref]
- Babu R, Adamson DC (2012) Fluorescence-guided malignant glioma resections. Curr Drug Discov Technol 9: 256-267. [crossref]
- Kaneko SA, Kaneko SA (2016) Fluorescence-Guided Resection of Malignant Glioma with 5-ALA. International Journal of Biomedical Imaging.
- Stupp R, Tonn JC, Brada M, Pentheroudakis G; ESMO Guidelines Working Group (2010) High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21: v190-193. [crossref]
- Georg Widhalm (2014) Intraoperative visualization of brain tumors with 5-aminolevulinic acid-induced fluorescence. Clinical Neuropathology Volume 33: 260 - 278.
- Shinoda J, Sakai N, Murase S, et al. (2001) Selection of eligible patients with supratentorial glioblastoma multiforme for gross total resection. J Neurooncol 52: 161-171.
- Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, et al. (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95: 190-198. [crossref]
- Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, et al. (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62: 564-576. [crossref]
- Tykocki T, Michalik R, Bonicki W, et al. (2012) Fluorescence-guided resection of primary and recurrent malignant gliomas with 5-aminolevulinic acid. Preliminary results. Neurol Neurochir Pol 46: 47-51.
- Hadjipanayis CG, Widhalm G, Stummer W (2015) What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 77: 663-673. [crossref]
- Wachowska, Malgorzata, et al. (2011)"Aminolevulinic acid (ALA) as a prodrug in photodynamic therapy of cancer." Molecules 16: 4140-4164.
- Martin Hefti, H Maximilian Meddorn, Ina Albert, Lutz Dorner (2010) Fluorescence-Guided surgery for Malignant Glioma: A Review on aminolevulinic acid Induced Protoporphyrin IX Photodyamic Diagnostic in Brain Tumors. Current Medical Imaging Reviews.
- Panciani PP, Fontanella M, Schatlo B, Garbossa D, Agnoletti A, et al. (2012) Fluorescence and image guided resection in high grade glioma. Clin Neurol Neurosurg 114: 37-41. [crossref]
- Baehring JM, Bi WL, Bannykh S, Piepmeier JM, Fulbright RK (2007) Diffusion MRI in the early diagnosis of malignant glioma. J Neurooncol 82: 221-225. [crossref]
- Michael Jenkinson (2011) Consultant Neurosurgeon. Walton Centre / Clatterbridge Centre for Oncology guidelines for high-grade glioma management. “High grade glioma guidelines”.
